
Concept explainers
Interpretation: To propose a plausible synthesis for
Concept introduction: The retrosynthesis begins with disconnection at the ether groups, at two of the
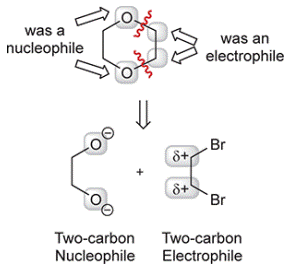
Each of these starting materials can be made from acetylene.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
ORGANIC CHEM PRINT STUDY GDE & SSM
- Ketones and aldehydes react with sodium acetylide (the sodium salt of acetylene) to give alcohols, as shown in the following example: R OH 1. HC=C: Na* 2. H30* R2 Rí `R2 HC Draw the structure of the major reaction product when the following compound reacts with sodium acetylide, assuming that the reaction takes preferentially from the Re face of the carbonyl group. • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • You do not have to explicitly draw H atoms. • If a group is achiral, do not use wedged or hashed bonds on it. H H3C H CH3arrow_forwardSuccinic acid can be synthesized by the following series of reactions from acetylene. Show the reagents and experimental conditions necessary to carry out this synthesis. НО OH НО. HO H- H. HO, ОН Acetylene 2-Butyne-1,4-diol 1,4-Butanediol Butanedioic acid (Succinic acid)arrow_forward2: By using the same alkene, how do you synthesize the following alcohols? Give the alkene and show the mechanisms for each product formation. OH OH HOarrow_forward
- Starting from acetylene and alkyl halides not longer than 4 carbon atoms, show the synthesis of 3-octanol and a ketone. Indicate the reagents in each step. You may use more than one mole of the reactants.arrow_forwardPlease help me with the last two steps. I keep getting them marked wrong. Please and thank you!arrow_forwardKetones and aldehydes are hydrated under acidic or basic conditions. An example of the acid-catalyzed hydration is shown. Complete the first step of this mechanism. Include all lone pairs of electrons, curved arrows, and nonzero formal charges in your mechanism.arrow_forward
- Methyllithium (CH3Li) is often used as a base in organic reactions. Treat any X−Li bond as X−Li+. Draw each of the products of the reaction of methyllithium with ethanol.arrow_forwardThe acid-catalyzed hydrolysis of an ester converts an ester into a carboxylic acid. Although there are two O atoms that can be protonated, the first step in the mechanism is believed to be protonation of the oxygen in the C=0 group. Based on charge stability, why is it favorable to protonate that oxygen? Hint: Draw out the products of each protonation. + Hо HO, НО Carboxylic acid Ester Alcoholarrow_forwardGive the product and mechanism for the following intramolecular reaction. Be sure to include all mechanism arrows, lone pairs, and formal charges. The product is an ether.arrow_forward
- Diazomethane, CH2N2, is used in the organic chemistry laboratory despite its danger because it produces very high yields and is selective for reaction with carboxylic acids. Write the products of the following reactions.arrow_forward#16d. Provide the missing reactants, reagents, or products for the following reaction sequences below.arrow_forwardYou are trying to determine whether the following organic reaction can be done in a single synthesis step. If so, add any missing reagents or conditions in the drawing area below. If it isn't possible to do this reaction in a single synthesis step, check the box below the drawing area instead. 口 OH : + T X Clarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

