
General, Organic, & Biological Chemistry
3rd Edition
ISBN: 9780073511245
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11.2, Problem 11.2P
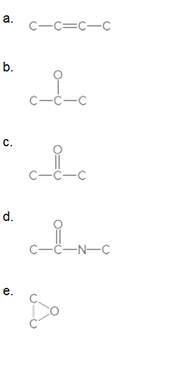
Fill in all H's and lone pairs in each compound.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which molecules show an appropriate number of bonds around each carbon atom?
Predict the geometry around each highlighted atom.
Draw in all the lone pairs on each structure.
Chapter 11 Solutions
General, Organic, & Biological Chemistry
Ch. 11.1 - Prob. 11.1PCh. 11.2 - Fill in all H's and lone pairs in each compound.Ch. 11.3 - Prob. 11.3PCh. 11.3 - Prob. 11.4PCh. 11.3 - Prob. 11.5PCh. 11.3 - Prob. 11.6PCh. 11.3 - How many lone pairs are present in lidocaine, the...Ch. 11.4 - Convert each compound to a condensed formula.Ch. 11.4 - Convert each condensed formula to a complete...Ch. 11.4 - Convert each skeletal structure to a complete...
Ch. 11.4 - How many H’s are bonded to each indicated carbon...Ch. 11.5 - Prob. 11.12PCh. 11.5 - For each compound: [1] Identify the functional...Ch. 11.5 - Prob. 11.14PCh. 11.5 - Prob. 11.15PCh. 11.5 - Prob. 11.16PCh. 11.5 - Identify all of the functional groups in atenolol,...Ch. 11.5 - Prob. 11.18PCh. 11.5 - Prob. 11.19PCh. 11.6 - Indicate the polar bonds in each compound. Label...Ch. 11.6 - Prob. 11.21PCh. 11.6 - Prob. 11.22PCh. 11.6 - Predict the water solubility of each compound....Ch. 11.6 - Prob. 11.24PCh. 11.7 - Prob. 11.25PCh. 11.7 - Prob. 11.26PCh. 11 - Prob. 11.27PCh. 11 - Prob. 11.28PCh. 11 - Complete each structure by filling in all H’s and...Ch. 11 - Prob. 11.30PCh. 11 - Prob. 11.31PCh. 11 - Prob. 11.32PCh. 11 - Prob. 11.33PCh. 11 - Prob. 11.34PCh. 11 - “Ecstasy” is a widely used illegal stimulant....Ch. 11 - Prob. 11.36PCh. 11 - Explain why each C—C—C bond angle in benzene...Ch. 11 - Prob. 11.38PCh. 11 - Prob. 11.39PCh. 11 - Prob. 11.40PCh. 11 - Convert each compound to a skeletal structure.Ch. 11 - Convert each compound to a skeletal structure.Ch. 11 - Convert each shorthand structure to a complete...Ch. 11 - Convert each shorthand structure to a complete...Ch. 11 - A and B are ball-and-stick models of two compounds...Ch. 11 - Prob. 11.46PCh. 11 - What is wrong in each of the following shorthand...Ch. 11 - Prob. 11.48PCh. 11 - Prob. 11.49PCh. 11 - Albuterol (trade names Proventil and Ventolin) is...Ch. 11 - Prob. 11.51PCh. 11 - Prob. 11.52PCh. 11 - Prob. 11.53PCh. 11 - Prob. 11.54PCh. 11 - Prob. 11.55PCh. 11 - GHB is an addictive, illegal recreational drug...Ch. 11 - Prob. 11.57PCh. 11 - Prob. 11.58PCh. 11 - Prob. 11.59PCh. 11 - Prob. 11.60PCh. 11 - Prob. 11.61PCh. 11 - Prob. 11.62PCh. 11 - You are given two unlabeled bottles of solids, one...Ch. 11 - State how potassium iodide (KI) and pentane...Ch. 11 - The given beaker contains 100 mL of the organic...Ch. 11 - Prob. 11.66PCh. 11 - Why do we need to know the shape of a molecule...Ch. 11 - 1,1-Dichloroethylene (CH2=CCl2) is a starting...Ch. 11 - Indicate the polar bonds in each molecule. Label...Ch. 11 - Indicate the polar bonds in each molecule. Label...Ch. 11 - Classify each molecule as polar or nonpolar.Ch. 11 - Classify each molecule as polar or nonpolar. a....Ch. 11 - Which molecule is more water soluble? Explain.Ch. 11 - Explain why pantothenic acid, vitamin B5, is water...Ch. 11 - Prob. 11.75PCh. 11 - Prob. 11.76PCh. 11 - Explain why regularly taking a large excess of a...Ch. 11 - You can obtain the minimum daily requirement of...Ch. 11 - Prob. 11.79PCh. 11 - Vitamin B6 is obtained by eating a diet that...Ch. 11 - Prob. 11.81PCh. 11 - Can an oxygen-containing organic compound, have...Ch. 11 - Prob. 11.83PCh. 11 - Prob. 11.84PCh. 11 - Benzocaine is the active ingredient in topical...Ch. 11 - Methyl salicylate is responsible for the...Ch. 11 - Answer the following questions about aldosterone,...Ch. 11 - Answer the following questions about...Ch. 11 - Prob. 11.89PCh. 11 - Skin moisturizers come in two types, (a) One type...Ch. 11 - THC is the active component in marijuana (Section...Ch. 11 - Cocaine is a widely abused, addicting drug....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please include all lone pairs and charges. thank you!arrow_forwardConvert each condensed formula to a Lewis structure.1.) (CH3)2CHOCH2CH2CH2OH2.) CH3(CH2)2CO2C(CH3)3arrow_forwardFor the following compound identify the lone pairs and indicate if each lone pair is localized or delocalized. Draw out the resonance structures.arrow_forward
- There are at least three different molecules with the formula C3H8O. Draw a Lewis structure for each possible constitutional isomer. Be careful not to duplicate any structures.arrow_forwardDraw a skeletal ("line") structure of this molecule: CH3 O CH-CH3 CH3 -C—CH—CH2−C—CH3arrow_forwardAdd lone pairs and draw 2 resonance structuresarrow_forward
- Draw in all hydrogens and lone pairs on the charged carbons in each ion ?arrow_forwardFor which compounds can a second resonance structure be drawn?Draw an additional resonance structure and the hybrid for eachresonance-stabilized compound.arrow_forwardConvert each skeletal structure to a complete structure with all C's, H's, and lone pairs drawn in.arrow_forward
- The compound above exists in the form of ________ isomer. a. a cis- b. a trans- c. both a cis- and a trans- d. neither a cis- nor a trans-arrow_forwardOn the worksheet, add any missing lone pairs.arrow_forwardConsidering structures A–D, classify each pair of compounds as isomers, resonance structures, or neither: (a) A and B; (b) A and C; (c) A and D; (d) B and D.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY