
Interpretation:
The amount of Aluminum required to be added and the liquid mixture cooled
diagram needs to be determined by referring to the given phase.
Concept introduction:
The aluminum alloy consists of aluminum as the parent material or the main constituent of the alloy. Aluminum alloy develops a white surface which acts as a protective layer of aluminum oxide.
Aluminum alloy has its major applications in the field of Aerospace industry as they are lighter, less flammable and have more strength.
Phase diagram is used to represent the
Answer to Problem 12.17P
The amount of aluminum to be added is calculated as
Explanation of Solution
Given Information:
Amount of aluminum
Weight percent of Cu-Al alloy
Calculation:
The composition of the Cu-Al alloy is Cu-
To calculate the temperature of the alloy, following expression is used,
Where, the alloy temperature=
The upper boundary temperature=
The lower boundary temperature=
Now, substituting the values as
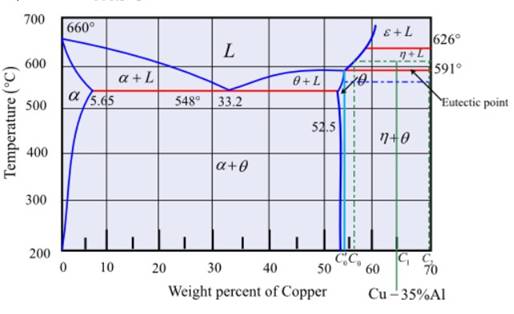
Fig.
In the above-mentioned phase diagram, the Cu-
To calculate the weight percent of the alloy at temperature
Where,
Substituting the values as
To calculate the weight percentage of the mentioned alloy at the eutectic temperature
Where
Substituting the values as
Now, to calculate the amount of the aluminum to be added more, the following formula is used:
Where
Substituting the values as,
Putting the values,
To calculate the temperature change,
Where the values substituted are
Thus, based on the phase diagram of Cu-
Want to see more full solutions like this?
Chapter 12 Solutions
ESS.MAT.SCI (LL W/MINDTAP)
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





