
(a)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 39E
Electron dot structure of
![]()
The structural formula of
![]()
Explanation of Solution
In molecule
![]()
Figure 1
![]()
Figure 2
Solid line, in Figure 2, between the iodine and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
(b)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 39E
Electron dot structure of
![]()
The structural formula of
![]()
Explanation of Solution
In molecule
![]()
Figure 3
![]()
Figure 4
Each solid line, in Figure 4, between the iodine and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the central atom iodine and the surrounding oxygen atom.
An electron dot structure and structural formula of
(c)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 39E
Electron dot structure of
![]()
The structural formula of
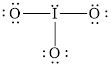
Explanation of Solution
In molecule
![]()
Figure 5

Figure 6
Each solid line, in Figure 6, between the iodine and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the central atom iodine and the surrounding oxygen atom.
An electron dot structure and structural formula of
(d)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 39E
Electron dot structure of
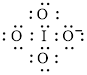
The structural formula of
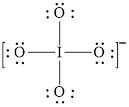
Explanation of Solution
In molecule

Figure 7

Figure 8
Each solid line, in Figure 8, between the iodine and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the central atom iodine and the surrounding oxygen atoms.
An electron dot structure and structural formula of
Want to see more full solutions like this?
Chapter 12 Solutions
Masteringchemistry With Pearson Etext -- Valuepack Access Card -- For Introductory Chemistry: Concepts And Critical Thinking
- Which of the following statements are true regarding a covalent bond between nitrogen and oxygen atoms in a nitric oxide, NO, molecule? (a) Valence electrons are shared between nitrogen and oxygen atoms. (b) Bonding electrons are found only between the bonded atoms. (c) The bond length is greater than the sum of the two atomic radii. (d) Energy is required to break a covalent bond.arrow_forwardThe atomic number of sulfur is 16. Sulfur combines withhydrogen by covalent bonding to form a compound, hydrogensulfide. Based on the number of valence electrons in a sulfuratom, predict the molecular formula of the compound.(A) HS(B) HS2(C) H2S(D) H4Sarrow_forwardWrite the chemical formula for the ionic compound formed from each pair of ions.(a) Mg2+ and I-(b) Na+ and O2-arrow_forward
- Answer true or false. (a) The name of a binary ionic compound consists of the name of the positive ion followed by the name of the negative ion. (b) In naming binary ionic compounds, it is necessary to state the number of each ion present in the compound. (c) The formula of aluminum oxide is Al2 O3 . (d) Both copper(II) oxide and cupric oxide are acceptable names for CuO. (e) The systematic name for Fe2 O3 is iron(II) oxide. (f) The systematic name for FeCO3 is iron carbonate. (g) The systematic name for NaH2PO4 is sodium di- hydrogen phosphate. (h) The systematic name for K2HPO4 is dipotassium hydrogen phosphate. (i) The systematic name for Na2O is sodium oxide. (j) The systematic name for PCl3 is potassium chloride. (k) The formula of ammonium carbonate is NH4CO3. 39. (a) A covalent bond is formed between two atoms whose difference in electronegativity is less than 1.9. (b) If the difference in electronegativity between two atoms is zero (they have identical electronegativ- ities),…arrow_forward2. Draw the Lewis structures for each of the following ions or molecules. Give the number of electrons in each species. Remember to enclose ions in square brackets with the charge as a superscript outside the right bracket. (a) Br 20 (c) BrF 3 (e) PCI 4* (b) IOF 3 (I is the central atom) (d) ASF 3arrow_forwardNa+ forms an “ionic bond” (i.e. an electrostatic attraction) with the OCN− ion. (a) Draw the full Lewis structure of the ionic compound. Be sure to show how you have derived this. (The ionic compound as a whole, not just OCN-) (b) Which atom in the OCN− anion is the sodium cation most likely to attract? Explain.arrow_forward
- Predict which of these compounds are ionic and which are covalent.(A) Ca3N2(B) Li2CO3(C) PCl5(D) NaOH(E) CH4(F) MgOarrow_forward(a) The 03 molecule has a central oxygen atom bonded to two outer oxygen atoms that are another. In the box below, draw the Lewis electron-dot diagram of the 03 molecule. Include all valid resonance structures. 0 - 0 = 0 (b) Based on the diagram you drew in part (a), what is the shape of the ozone molecule? and trigonal Bent Ozone decomposes according to the reaction represented below. 2 03(g) → 3 0₂(8) (c) The bond enthalpy of the oxygen-oxygen bond in O₂ is 498 kJ/mol. Based on the enthalpy of the reaction represented above, what is the average bond enthalpy, in kJ/mol, of an oxygen-oxygen bond in 03 ? Ozone can oxidize HSO3(aq), as represented by the equation below. [0] 1.0 x 10-5. <-> 00: HSO3(aq) + O3(aq) → HSO4 (aq) + O₂(8) A solution is prepared in which the initial concentration of HSO₂ (aq) (6.4 × 10+ M) is much larger than that of O3(aq) (1.0 × 10-5 M). The concentration of O3(aq) is monitored as the reaction proceeds, and the data are plotted in the graph below. 8.0 x…arrow_forwardA resident expert on electronegativity comes up to visit with you. He makes two claims (seen below) about electronegativity with relation to covalent bonding. Is the expert correct or can you refute him with your knowledge of electronegativity? (a) If a diatomic molecule is made up of atoms X and Y, which have different electronegativities, the molecule must be polar. (b) The farther two atoms are apart in a bond, the larger the dipole moment will be.arrow_forward
- Consider the following compounds: BeCl 2 , MgBr 2 , and SrBr 2 . Answer the following questions based on expected periodic trends: (a) Which is expected to have the shortest ionic bonds? (b) Which is expected to have the highest lattice energy? (c) Which is expected to have the lowest melting point?arrow_forwardUsing Lewis electron-dot symbols to depict the monatomic ions formed from each of the following reactants, predict the formula of the compound the ions produce.(Type your answer using the format CO2 for CO2.) (a) O and Ca (b) N and Mg (c) Br and Li (d) K and Parrow_forwardii. Answer true or false. (a) A covalent bond is formed between two atoms whose difference in electronegativity is less than 1.9. (b) If the difference in electronegativity between two atoms is zero (they have identical electronegativities), then the two atoms will not form a covalent bond. (c) A covalent bond formed by sharing two electrons is called a double bond. (d) In the hydrogen molecule (H2), the shared pair of electrons completes the valence shell of each hydrogen. (e) In the molecule CH4, each hydrogen has an electron configuration like that of helium, and carbon has an electron configuration like that of neon. (f) In a polar covalent bond, the more electronegative atom has a partial negative charge (8-) and the less electronegative atom has a partial positive charge (&+). (g) These bonds are arranged in order of increasing polarity C-Harrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
