
Concept explainers
(a)
Interpretation:The name of the following compound should be determined:
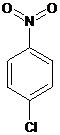
Concept Introduction: International Union of Pure and Applied Chemistry (IUPAC) recommended a nomenclature method used for naming organic chemical compounds.
(a)
Answer to Problem 59P
1-chloro-4-nitrobenzene.
Explanation of Solution
For the given organic compound, the number of substituents present on benzene ring is two and no substituent in the compound imparts special name. So, the numbering is done according to alphabetical order:
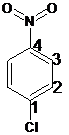
The name of the substituent at position 1 is chloro and at position 4 is nitro. So, the name of the compound is 1-chloro-4-nitrobenzene.
(b)
Interpretation: The name of the following compound should be determined:
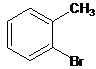
Concept Introduction: International Union of Pure and Applied Chemistry (IUPAC) recommended a nomenclature method used for naming organic chemical compounds.
(b)
Answer to Problem 59P
2-bromotoluene.
Explanation of Solution
For the given organic compound, the number of substituents present on benzene ring is two and due to presence of methyl group its parent molecule is -toluene. The numbering for rest substituent is done as shown:
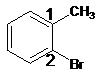
The name of the substituent is -bromo and is present at position 2 so, the name of the compound is 2-bromotoluene.
(c)
Interpretation: The name of the following compound should be determined:
Concept Introduction: International Union of Pure and Applied Chemistry (IUPAC) recommended a nomenclature method used for naming organic chemical compounds.
(c)
Answer to Problem 59P
1-chloro-3-phenylpropane.
Explanation of Solution
In
The substituent at position 1 is chloro so, the name of the compound is 1-chloro-3-phenylpropane.
(d)
Interpretation: The name of the following compound should be determined:
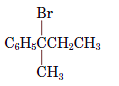
Concept Introduction: International Union of Pure and Applied Chemistry (IUPAC) recommended a nomenclature method used for naming organic chemical compounds.
(d)
Answer to Problem 59P
2-bromo-2-phenylbutane.
Explanation of Solution
In the given compound,
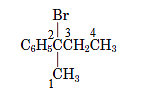
The substituent at position 2 is bromo so, the name of the compound is 2-bromo-2-phenylbutane.
(e)
Interpretation: The name of the following compound should be determined:
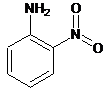
Concept Introduction: International Union of Pure and Applied Chemistry (IUPAC) recommended a nomenclature method used for naming organic chemical compounds.
(e)
Answer to Problem 59P
2-nitroaniline.
Explanation of Solution
For the given organic compound, the number of substituents present on benzene ring is two and due to presence of
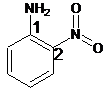
The name of the substituent is -nitro present at position 2 so, the name of the compound is 2-nitroaniline.
(f)
Interpretation: The name of the following compound should be determined:
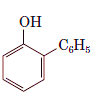
Concept Introduction: International Union of Pure and Applied Chemistry (IUPAC) recommended a nomenclature method used for naming organic chemical compounds.
(f)
Answer to Problem 59P
2-phenylphenol.
Explanation of Solution
For the given organic compound, the number of substituents present on benzene ring is two and due to presence of hydroxyl group its parent molecule is -phenol. The numbering for the substituent is done as shown:
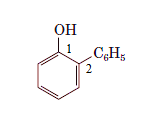
The name of the substituent is -phenyl present at position 2 so, the name of the compound is 2-phenylphenol.
(g)
Interpretation: The name of the following compound should be determined:
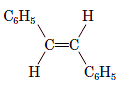
Concept Introduction: International Union of Pure and Applied Chemistry (IUPAC) recommended a nomenclature method used for naming organic chemical compounds.
(g)
Answer to Problem 59P
trans -1,2-diphenylethene.
Explanation of Solution
In the given compound,
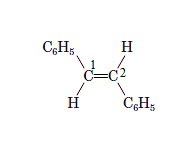
The phenyl substituents are present at opposite sides of the double bond so, the name of the compound istrans -1,2-Diphenylethene
(h)
Interpretation: The name of the following compound should be determined:

Concept Introduction: International Union of Pure and Applied Chemistry (IUPAC) recommended a nomenclature method used for naming organic chemical compounds.
(h)
Answer to Problem 59P
2,4-dichlorotoluene.
Explanation of Solution
For the given organic compound, the number of substituents present on benzene ring are three and due to presence of methyl group its parent molecule is -toluene. The numbering for the substituent is done as shown:
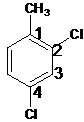
The name of the substituent is -chloro present at position 2 and 4 so, the name of the compound is 2,4-dichlorotoluene.
Want to see more full solutions like this?
Chapter 12 Solutions
Introduction to General, Organic and Biochemistry
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning



