
ORGANIC CHEMISTRY MASTERINGCHEM ACCESS
9th Edition
ISBN: 9780137249442
Author: Wade
Publisher: INTER PEAR
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.15A, Problem 12.9P
Show the fragmentations that give rise to the peaks at m/z 43, 57, and 85 in the mass spectrum of 2,4-dimethylpentane (Figure12-17).
| m/z | Abundance (% of base Peak) |
| 41 | 34 |
| 42 | 24 |
| 43(base peak) | 100 |
| 56 | 35 |
| 57 | 72 |
| 85 | 19 |
| 100 (M+) |
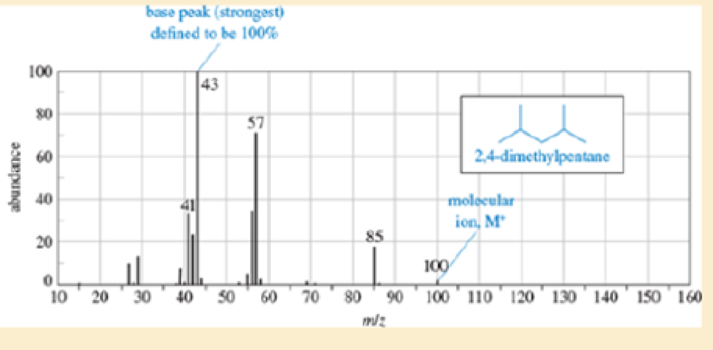
Figure 12-17
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The intensity of M+, M+1, and M+2 peak are 100.0, 6.8, 31.9. How many carbons
are in the compound? (whole number digit only)
100
MS-NW-5495
80
60
40
10
20
30
40
50
60
70
80
90
100
110
m/z
Relative Intensity
20
12. The mass spectrum of 2-methylhexane is shown below. What is the m/z value of the M*
peak and of the base peak? Give possible structures of the fragments giving rise to the
large peaks at m/z = 85,57, and 43.
100 -
80 -
40
20
50
60
70
80
90
100
10
20
30
40
m/z
Relative Intensity
Which of the following structures corresponds to the mass spectrum shown below?
Relative Intensity
100
80-
60
40
20
MS-NW-0358
0-mt
20
40
CI
60
80
100
120
m/z
140
160 180
M+ = 216
200 220
Chapter 12 Solutions
ORGANIC CHEMISTRY MASTERINGCHEM ACCESS
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The mass spectrum of 4-methoxybenzaldehyde B is shown in Figure 1. Assign the labelled signals and state whether each of your assignments is a fragment ion or a molecular ion. 4- methoxybenzaldehyde MASS SPECTRUM 100 136 80 60 78 108 40 20 0.0+ 0.0 40 80 120 160 m/z Figure 1 Rel. Intensityarrow_forwardThe following mass spectrum has a molecular ion and the M+2 ion. These molecular ions indicate the presence of a specific atom in the molecule. Identify this atom. Relative Intensity 100 80 8 40 20 0 ON 13C Br OCI 10 MS-NW-1039 20 30 40 50 60 m/z 70 80 90 100arrow_forwardAn unknown molecule was run through WT's mass spectrometer and the following spectra was produce. What could the unknown compound be? MASS SPECTRUM 100 50 80- 43 60 98 40 27 20- 83 0.0 0.0 20 40 60 80 100 m/z Rel. Intensityarrow_forward
- Loss of which fragment from the parent molecule could account for the peak at 57 in the mass spectrum of 2-methylbutane? 100 -43 57 80 29 100 110 Relative Intensity 60 20 0 T T T T T T T 10 ZD 30 40 50 60 72 70 m/z 80 90 120arrow_forwardIdentify the following in the given IR Mass spectrum. Compound 1 Mass Spectroscopy: Interpretation of peaks: Compound 1 Mass Spectroscopy: MASS SPECTRUM 100 80 60 40 20 0.0- 10 20 30 40 50 60 70 m/z Interpretation of peaks: Rel. Intensityarrow_forwarda) b) Acidity of Alcohols, Phenols and Thiols Predicting Acidity Rank the following compounds in order of increasing acidity: 1 1 OH OH Rel. Intensity 100 80 60 40 20 OH 0.0 2 0.0 2 OH Alcohols and Ethers Interpreting a Mass Spectrum give a peak at m/z = 45? 20 O 3 40 3 Example: Propose a structure for the following alcohol using the mass spectrum below (NOTE: there is a very small peak at m/z = 88) unknown MASS SPECTRUM OH LOH m/z 4 3 60 Hints: This is an alcohol, it has a mass of 88 so first determine the formula, then draw the possible structures. Now, which structure will fragment to OH OH 80 4 100arrow_forward
- The mass spectrum of 2,2-dimethylpropane shows only a very weak molecular ion peak at m/z = 72. However, a large peak at mlz = 57 is seen. Suggest a possible structure of the fragment giving rise to this large peak and suggest a reason as to why this peak is so large. For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). T U Paragraph 5arrow_forward5. Use the mass spectrum to propose a molecular formula for the compound. Show your work. 100 80 60 (M)* m/z = 87 intensity = 35% 40 (M+1) m/z = 88 intensity = 2% 20 0.0 0.0 20 40 60 80 100 m/z Rel. Intensityarrow_forwardwill be the pattern of the molecular ion peak(s) in the mass spectrum of 3-chloro-methyl-benzene (m-chloro-toluene). M+ at 126; largest peak at 76 M+ at 128; M+2 at 130, ratio 3:1 M+ at 128; M+2 at 130; ration 1:1 M+ at 128 M+ at 126; M+2 at 128, ratio 3:1 QUESTION 14 In a recrystallization, the solute is dissolved in solvent. cold room temperature hot QUESTION 15 Consider the aromatic compound 4-isopropyl-benzonițrile. (Benzonitrile is a benzene ring with the nitrile group on position 1.) How many for non-equivalent types of protons will be in its proton NMR spectrum? 4. 6arrow_forward
- QUESTION 27 will be the pattern of the molecular ion peak(s) in the mass spectrum of 3-chloro-methyl-benzene (m-chloro-toluene). O M+ at 126; largest peak at 76 O M+ at 128; M+2 at 130, ratio 3:1 O M+ at 128; M+2 at 130; ration 1:1 M+ at 128 M+ at 126; M+2 at 128, ratio 3:1arrow_forwardQ1/ What is the possible structural formula for a compound that contains the elements (C, H, O) and has the Following spectroscopic results with the interpretation of these results: 11 TRANSMITTANCE Rel. Intensity 10 100 0.8 0.6 0.4 80 0.0 0.2 60 40 20 0.0 4000 9 2 8 Solvent 3000 M.wt = 136 M+1 = 8.8 M+2 = 0.78 da 2 40 7 6 80 m/z Wavenumber (cm-1) 5 8 (ppm) 2000 120 3 3 1000 160arrow_forwardFollowing is the mass spectrum of an unknown compound. The two highest peaks are at mlz 120 and 122. Suggest a structure for this compound. (Data from http://webbook .nist.gov/chemistry/.) 100 41 80 20 120 122 0 rt 10 20 30 40 60 70 110 140 80 90 m/z 50 100 120 130 150 160 Relative Abundancearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY