
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.9, Problem 8P
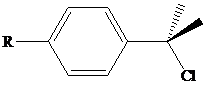
As measured by their first-order rate constants, the compound shown (
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain the following result. Although alkenes are generally more reactive than alkynes toward electrophiles, the reaction of Cl2 with but-2-yne can be stopped after one equivalent of Cl2 has been added.
Explain the following result. Although alkenes are generally more reactive than alkynes towards electrophiles, the reaction of Cl2 with but-2-yne can be stopped after one equivalent of Cl2 has been added.
what structure show the transition state for the rate determining step in the sn1 reaction with this picture?
Chapter 12 Solutions
Organic Chemistry - Standalone book
Ch. 12.2 - Write structural formulas for toluene (C6H5CH3)...Ch. 12.3 - Prob. 2PCh. 12.5 - Prob. 3PCh. 12.5 - Prob. 4PCh. 12.6 - Prob. 5PCh. 12.6 - Chrysene is an aromatic hydrocarbon found in coal...Ch. 12.8 - Prob. 7PCh. 12.9 - As measured by their first-order rate constants,...Ch. 12.9 - Give the structure of the principal organic...Ch. 12.9 - Prob. 10P
Ch. 12.10 - Prob. 11PCh. 12.11 - Prob. 12PCh. 12.12 - Prob. 13PCh. 12.13 - Prob. 14PCh. 12.13 - Prob. 15PCh. 12.15 - The regioselectivity of Birch reduction of...Ch. 12.16 - Prob. 17PCh. 12.17 - Both cyclooctatetraene and styrene have the...Ch. 12.17 - Prob. 19PCh. 12.18 - Give an explanation for each of the following...Ch. 12.19 - Prob. 21PCh. 12.19 - What does a comparison of the heats of combustion...Ch. 12.20 - Prob. 23PCh. 12.20 - Prob. 24PCh. 12.20 - Prob. 25PCh. 12.20 - Prob. 26PCh. 12.20 - Prob. 27PCh. 12.20 - Prob. 28PCh. 12.21 - Prob. 29PCh. 12.21 - Prob. 30PCh. 12.22 - Prob. 31PCh. 12.22 - Prob. 32PCh. 12 - Write structural formulas and give the IUPAC names...Ch. 12 - Prob. 34PCh. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - Prob. 37PCh. 12 - Acridine is a heterocyclic aromatic compound...Ch. 12 - Prob. 39PCh. 12 - Prob. 40PCh. 12 - Prob. 41PCh. 12 - Evaluate each of the following processes applied...Ch. 12 - Prob. 43PCh. 12 - Prob. 44PCh. 12 - Prob. 45PCh. 12 - Prob. 46PCh. 12 - Anthracene undergoes a DielsAlder reaction with...Ch. 12 - Prob. 48PCh. 12 - Prob. 49PCh. 12 - The relative rates of reaction of ethane, toluene,...Ch. 12 - Both 1,2-dihydronaphthalene and...Ch. 12 - Prob. 52PCh. 12 - Prob. 53PCh. 12 - Prob. 54PCh. 12 - Prob. 55PCh. 12 - Prob. 56PCh. 12 - Each of the following reactions has been described...Ch. 12 - Prob. 58PCh. 12 - A compound was obtained from a natural product and...Ch. 12 - Prob. 60PCh. 12 - Suggest reagents suitable for carrying out each of...Ch. 12 - Prob. 62PCh. 12 - Prob. 63DSPCh. 12 - Prob. 64DSPCh. 12 - Prob. 65DSPCh. 12 - Prob. 66DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q4 Which statement below about Sn1 reactions is incorrect? (A) SN1 reactions are stepwise and have intermediates. (B) The slow step in a SN1 reaction is formation of the carbocation intermediate. (C) SN1 reactions have first order kinetics which means only the alkyl halide is involved in the rate limiting step. (D) The products of a SN1 reaction will be a pair of enantiomers. (E) An aprotic solvent is best for Sn1 reactions as they tend to help stabilize carbocation intermediates.arrow_forwardList the following order of increasing reactivity in an SN1 reactionarrow_forwardIn parts 1 and 2 draw the two organic products of this reaction, showing any nonzero formal charges. Then, in part 3 answer the question regarding purification of the reaction mixture. 1. Draw the product with the higher molecular weight here: 2. Draw the product with the lower molecular weight here:arrow_forward
- Propose a mechanism for this reaction. Account for both its regioselectivity and stereoselectivity.arrow_forwardWhat products will be obtained from the E1 reaction of the alkyl halides in Problem 45?arrow_forwardThe reaction of one equivalent of hydrogen bromide with 1,3-butadiene gives product D and G via pathway 1 and 2 respectively. Which pathway has a faster rate of reaction?arrow_forward
- Complete the reactions below, write the type of mechanism (S1, S2, E2).arrow_forwardComplete the reactions given below, write down the type of mechanism (SN1, SN2, E1, E2)?arrow_forwardWhat happens to the rate of an SN1 reaction under the following conditions? [RX] is halved, and [:Nu−] is doubledarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License