
Concept explainers
The mass spectrum of the following compound shows fragments at

Interpretation: The structures of the ions of a given compound that shows fragments at
Concept introduction: Molecular ion is the radical cation which is formed by ejection of electrons from a molecule when a beam of high-energy electrons bombarded on a molecule. Mass of the molecule is equal to
Answer to Problem 15P
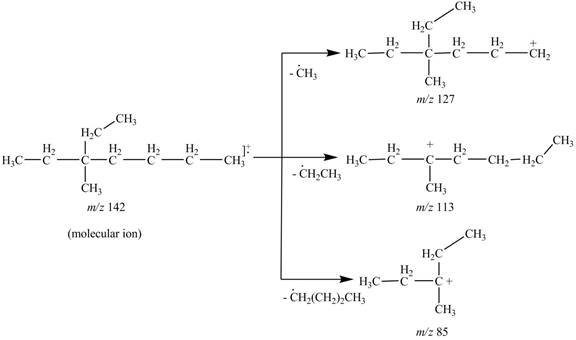
The fragments of 3-ethyl, 3-methylheptane at
Explanation of Solution
The ball and stick model of the given compound is,

Figure 1
Black colored atoms have four bonds. So, these are the carbon atoms. The grey colored balls have one bond. So, these are the hydrogen atoms. The molecular structure is,
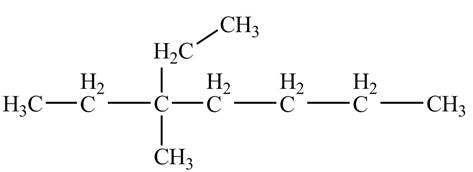
Figure 2
Hence, the ball and stick model of the given compound is 3-ethyl, 3-methylheptane. The mass of 3-ethyl, 3-methylheptane is follow.
Thus, the molecular ion peak of 3-ethyl, 3-methylheptane is observed at
The molecular ion
The molecular ion
The molecular ion
Hence, the fragments of 3-ethyl, 3-methylheptane at
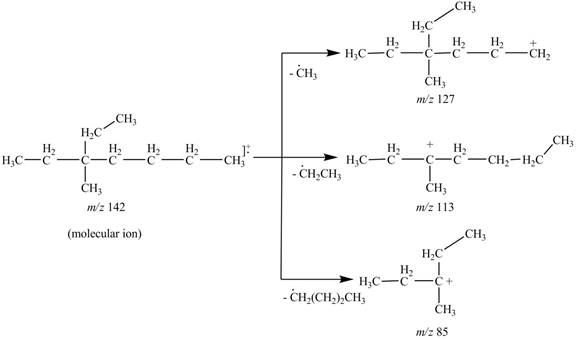
Figure 3
The fragments of 3-ethyl, 3-methylheptane at
Want to see more full solutions like this?
Chapter 12A Solutions
ORGANIC CHEMISTRY (LL+SM+ACCESS)
Additional Science Textbook Solutions
General, Organic, & Biological Chemistry
Chemistry For Changing Times (14th Edition)
Chemistry & Chemical Reactivity
Chemistry
Organic Chemistry As a Second Language: Second Semester Topics
Organic Chemistry - Standalone book
- Following is the mass spectrum of an unknown compound. The two highest peaks are at m/z 120 and 122. Suggest a structure for this compound. (Data from http://webbook.nist.gov/chemistry/.)arrow_forwardPropose structures for compounds that fit the following mass-spectral data: (a) A hydrocarbon with M+=132 (b) A hydrocarbon with M+=166 (c) A hydrocarbon with M+=84arrow_forwardThe mass spectrum (a) and the infrared spectrum (b) of an unknown hydrocarbon are shown. Propose as many structures as you can.arrow_forward
- Write molecular formulas for compounds that show the following molecular ions in their high-resolution mass spectra, assuming that C, H, N, and O might be present. The exact atomic masses are: 1.007 83 (1H), 12.000 00 (12C), 14.003 07 (14N), 15.994 91 (16O). (a) M+=98.0844 (b) M+=123.0320arrow_forwardThe mass spectrum (a) and the infrared spectrum (b) of another unknown hydrocarbon are shown. Propose as many structures as you can.arrow_forwardThe mass spectrum of compound A shows the molecular ion at m/z 85, an M + 1 peak at m/z 86 of approximately 6% abundance relative to M, and an M + 2 peak at m/z 87 of less than 0.1% abundance relative to M. Q.) Propose a molecular formula for compound A.arrow_forward
- Is the structure on the bottom right corner of the FTIR photo correct for the mass spectrum, FTIR, and a proton NMR that has a doublet with an integration of 3 hydrogens at 7.25 ppm, a triplet with an integration of 1 hydrogen at 7.41 ppm, and a singlet with an integration of 6 hydrogen at 2.6 ppm? If not, what is the correct structure?arrow_forwardA mass spectrum shows significant peaks at m/z = 87, 115, 140, and 143. Which of the following compounds is responsible for that mass spectrum?arrow_forwardThe molecular ion in the mass spectrum of 2-methyl-1-pentene appears at m/z 84. Propose structural formulas for the prominent peaks at m/z 69, 55, 41, and 29.arrow_forward
- The mass spectrum of the following compound shows fragments at m/z= 127, 113, and 85. Propose structures for the ions that give rise to thesepeaks.arrow_forwardPropose a molecular formula for a compound that exhibits the following peaks in its mass spectrum. (M)+• at m/z = 68, relative height = 100% (base peak) (M+1)+• at m/z = 69, relative height = 4.3%arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

