
Concept explainers
13.106 Hydrazine,
(a) Which reaction occurs at the anode and which at the cathode?
(b) What is the net cell reaction?
(C) If the cell is to produce 0.50 A of current for 50.0 h, what mass in grams of hydrazine must be present?
(d) What mass in grams of
(b)
Interpretation:
To identify the net reaction.
Concept introduction:
- The net reaction is the total reaction including all reactants and products.
- It is obtained via addition of the half reactions.
Answer to Problem 13.106PAE
Solution:
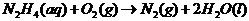
Explanation of Solution
In order to find out the reaction, we must include the reduction-oxidation reactions. They always take place together, since one donates electrons and the other receives them.
In this case, the oxidation reaction: hydrazine oxidation to nitrogen gas. The reduction is oxygen forming hydroxide ion via the addition of electron
Adding both reactions, getting rid of repeating/same units on the left/right side of the equation:
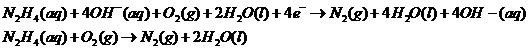
Therefore, the reaction taking place is:
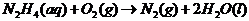
(c)
Interpretation:
To calculate the mass of hydrazine present in given amount of time and current.
Concept introduction:
- A stands for ampere, the amount of current.
- Time and current can be used to calculate total charge.
- The total charge relates mol of e- and Faraday constant.
Answer to Problem 13.106PAE
Solution:
Explanation of Solution
This consist of the following steps:
1st Step: Calculate the total amount of charge.
2nd Step: Calculate the total amount of moles of hydrazine
3rd Step: Calculate the mass of hydrazine
(d)
Interpretation:
To calculate the mass oxygen required
Concept introduction:
- Use stoichiometric ratios.
Answer to Problem 13.106PAE
Solution:
Explanation of Solution
Relate the moles of oxygen and hydrazine:
1st Step: Calculate total moles of oxygen required
The ratio is 1:1 since 1 mol of hydrazine requires 1 mol of Oxygen gas to react.
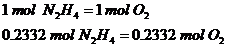
2nd Step: Calculate the total mass of oxygen gas
The mass is exactly the same as hydrazine since they have 1:1 ratio and similar molar masses.
Want to see more full solutions like this?
Chapter 13 Solutions
CHEM FOR ENG >I<>CUSTOM<
- Calcium metal can be obtained by the direct electrolysis of molten CaCl2, at a voltage of 3.2 V. (a) How many joules of electrical energy are required to obtain 12.0 1b of calcium? (b) What is the cost of the electrical energy obtained in (a) if electrical energy is sold at the rate of nine cents per kilowatt hour?arrow_forwardHydrazine, N2H4, has been proposed as the fuel in a fuel cell in which oxygen is the oxidizing agent. The reactions are N2H4(aq) + 4 OH(aq) N2(g) + 4 H2O() + 4e O2(g) + 2 H2O() + 4e 4 OH(aq) (a) Which reaction occurs at the anode and which at thecathode? (b) What is the overall cell reaction? (c) If the cell is to produce 0.50 A of current for 50.0 h, calculate what mass in grams of hydrazine must be present. (d) Calculate what mass (g) of O2 must be available to reactwith the mass of N2H4 determined in part (c).arrow_forwardAn electrode is prepared from liquid mercury in contact with a saturated solution of mercury(I) chloride, Hg2Cl, containing 1.00 M Cl . The cell potential of the voltaic cell constructed by connecting this electrode as the cathode to the standard hydrogen half-cell as the anode is 0.268 V. What is the solubility product of mercury(I) chloride?arrow_forward
- In the commercial preparation of aluminum, aluminum oxide, Al2O3, is electrolyzed at 1000C. (The mineral cryolite is added as a solvent.) Assume that the cathode reaction is Al3+3eAl How many coulombs of electricity are required to give 3.9 kg of aluminum?arrow_forwardChlorine, Cl2, is produced commercially by the electrolysis of aqueous sodium chloride. The anode reaction is 2Cl(aq)Cl2(g)+2e How long will it take to produce 2.00 kg of chlorine if the current is 5.00 102 A?arrow_forwardConsider the electrolysis of water in the presence of very dilute H2SO4. What species is produced at the anode? Atthe cathode? What are the relative amounts of the speciesproduced at the two electrodes?arrow_forward
- Aluminum is produced commercially by the electrolysis of Al2O3 in the presence of a molten salt. If a plant has a continuous capacity of 1.00 million A, what mass of aluminum can be produced in 2.00 h?arrow_forwardAn aqueous solution of an unknown salt of gold is electrolyzed by a current of 2.75 amps for 3.39 hours. The electroplating is carried out with an efficiency of 93.0%, resulting in a deposit of 21.221 g of gold. a How many faradays are required to deposit the gold? b What is the charge on the gold ions (based on your calculations)?arrow_forwardAssume the following electrochemical cell simulates the galvanic cell formed by copper and zinc in seawater at pH 7.90 and 25 C. Zn | Zn(OH)2(s) | OH(aq) || Cu(OH)2(s) | Cu(s) a. Write a balanced equation for the reaction that occurs at the cathode. b. Write a balanced equation for the reaction that occurs at the anode. c. Write a balanced chemical equation for the overall reaction. d. Determine the potential (in volts) of the cell.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





