
Anethole, the major constituent of anise oil, is used in licorice-flavored sweets and flavored brandy. Answer the following questions using the ball-and-stick model of anethole.
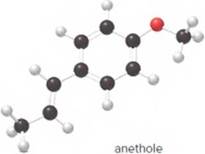
- What is the molecular formula of anethole?
(a)
Interpretation:
The molecular formula of anethole should be determined.
Concept Introduction:
The numbers and kinds of atoms present in a molecule of a compound are given by a formula said to be a molecular formula. This formula of a molecule gives the definite number of atoms of each element present in the compound.
Answer to Problem 13.29P
The molecular formula of anethole is C10H12O.
Explanation of Solution
The given ball-and-stick model of anethole is:
In ball-and-stick model, the black ball represents carbon, C atom, white ball represents hydrogen, H atom, and red ball represents oxygen, O atom.
The number of each atom in the ball-and-stick model is:
Carbon atoms = 10
Hydrogen atoms = 12
Oxygen atom = 1
Thus, the molecular of anethole is C10H12O.
(b)
Interpretation:
The functional group(s) in anethole should be determined.
Concept Introduction:
A group of atoms or an atom which is responsible for the characteristic reactions of a particular compound is said to be the functional group. Every functional group shows distinctive chemical properties irrespective to the moiety to which it is attached.
Answer to Problem 13.29P
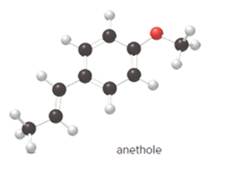
The functional group(s) in anethole is ether.
Explanation of Solution
The given ball-and-stick model of anethole is:
When the oxygen atom is connected to two alkyl or aryl groups with general formula R-O-R' such class of compounds is known as ether.
Since, in anethole compound the oxygen atom is bonded to an aryl and an alkyl group so, the functional group(s) in anethole is ether.
(c)
Interpretation:
The carbon-carbon double bond in anethole should be labeled as cis or trans.
Concept Introduction:
The molecule with same molecular formula and connectivity but differ in spatial arrangements of atoms in the molecule are said to be geometric isomers of each other.
Answer to Problem 13.29P
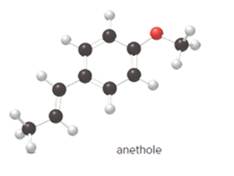
The carbon-carbon double bond in anethole is labeled as trans.
Explanation of Solution
The given ball-and-stick model of anethole is:
Geometric isomerism, or cis-trans, isomerism is most common in alkenes. The two forms exist due to no free rotation about carbon-carbon double bond. It exists only when the double bonded carbon atoms are joined to two different groups or atoms.
In cis- isomer, the two identical groups or atoms are close to each other whereas in trans- isomer two identical groups or atoms are farther apart.
Since, in the given molecule the groups attached to carbon-carbon double bonded are farther apart from each other so, the carbon-carbon double bond in anethole is trans.
(d)
Interpretation:
The structure of anethole should be drawn and each carbon should be labeled as trigonal planar or tetrahedral.
Concept Introduction:
In structural formula, the bonding and type of bonds which holds the atoms in molecule together are shown.
Answer to Problem 13.29P
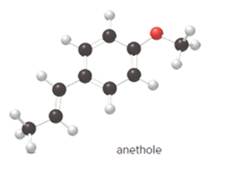
The structure of anethole is:
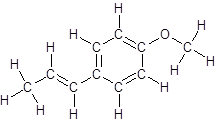
Labelling of each carbon is:
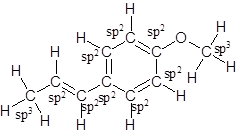
Explanation of Solution
The given ball-and-stick model of anethole is:
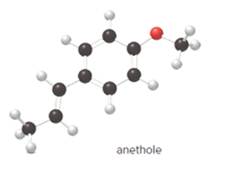
In ball-and-stick model, the black ball represents carbon, C atom, white ball represents hydrogen, H atom, and red ball represents oxygen, O atom.
So, the structure of anethole is:
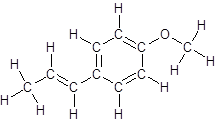
In the structure of anethole, there are 10 C atoms, 2 of which them are in CH3- groups which has four electron groups around the central carbon atom, so the electron group arrangement is tetrahedral, sp3hybridized. The 8 carbon atoms have three electron groups around the central carbon atom, so the electron group arrangement is trigonal planar, sp2hybridized.
Thus, the electron group arrangement of all the 10 carbon atoms in anethole is:
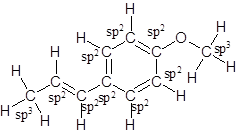
Want to see more full solutions like this?
Chapter 13 Solutions
General, Organic, & Biological Chemistry
- identify the functional group of each compound.arrow_forwardFor each of the following questions, give an example and correctly name the compoundyou gave:a. An octane molecule that contains a primary halide.b. A molecule that contains 6 carbon atoms with a single functional group that is analcohol.c. A molecule that contains a double bond and an ethyl side chain.d. An aromatic molecule that contains a tertiary halide.arrow_forwardDraw the skeletal ("line") structure of a secondary alcohol with 5 carbon atoms,1 oxygen atom, at least one ring, and no double or triple bonds.arrow_forward
- 1. How many total covalent bonds are present in the compound? (Please refer to the first image attached.) A. 8 B. 10 C. 12 D. 16 2. What is the correct IUPAC name for this compound? (Please refer to the second image attached.) A. 2,5,5-Trimethylhexane B. 2,2,5-Trimethylhexane C. 2,2,5-methylhexane D. 2,5,5-methylhexane 3. Which of the following is a tertiary alcohol? A. 2-Pentanol B. 2-Methyl-2-butanol C. 1-Butanol D. 2-Methyl-1-pentanolarrow_forwardEncircle the functional groups, label each functional group with a letter, and identify the type or class of compounds represented by each functional group. 3. Propanolol, a cardiac depressantarrow_forward1. Discuss the intermolecular forces involved in organic compounds.2. List all the structural formula of hexane and indicate its IUPAC name.3. Draw the expanded formula of the following compound:(a) 4-Ethyl-3,4-dimethyloctanearrow_forward
- Many naturally occurring compounds contain more than one functional group. Identify the functional groups in the following compounds:(a) Penicillin G is a naturally occurring antibiotic.(b) Dopamine is the neurotransmitter that is deficient in Parkinson’s disease.(c) Capsaicin gives the fiery taste to chili peppers.(d) Thyroxine is the principal thyroid hormone.(e) Testosterone is a male sex hormone.arrow_forwardIndicate whether each statement is true or false: The methyl group contains one less hydrogen atom than methane.arrow_forwardIn the models shown here, C atoms are black and H atoms are light blue: a Write the molecular formula of each molecule. b Write the condensed structural formula for each molecule. c Give the IUPAC name of each molecule.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





