
Concept explainers
(a)
Interpretation:
The structure of the compound having molecular formula,
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an
Answer to Problem 13.40AP
The structure of the compound having molecular formula,
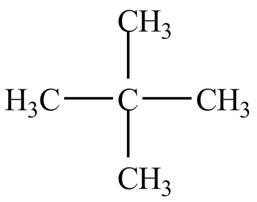
Explanation of Solution
There is only one signal in the compound that means all the protons are equivalent and all the protons are from methyl group as per chemical shift value of
Therefore, the structure of the compound corresponding to NMR data is shown below.
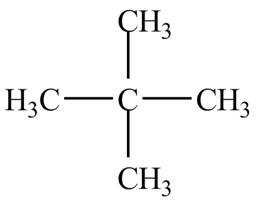
Figure 1
The structure of the compound having molecular formula,
(b)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
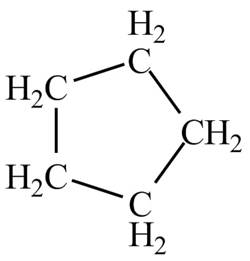
Explanation of Solution
There is only one signal in the compound that means all the protons are equivalent and all the protons are from methylene group as per chemical shift value of
Therefore, the structure of the compound corresponding to NMR data is shown below.
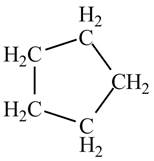
Figure 2
The structure of the compound having molecular formula
(c)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
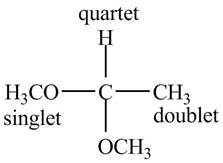
Explanation of Solution
There are three signals in the compound. One is at
Therefore, the structure of the compound corresponding to NMR data is shown below.

Figure 3
The structure of the compound having molecular formula
(d)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
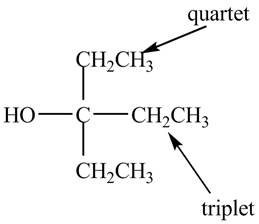
Explanation of Solution
The given spectrum is shown below.
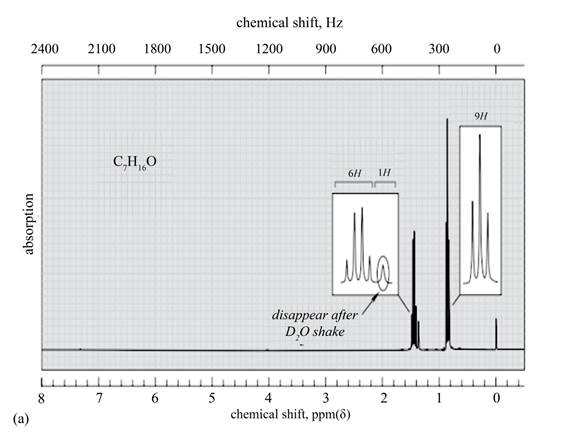
Figure 4
There are three signals in the compound. One is at
Therefore, the structure of the compound corresponding to NMR data is shown below.

Figure 5
The structure of the compound having molecular formula
(e)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
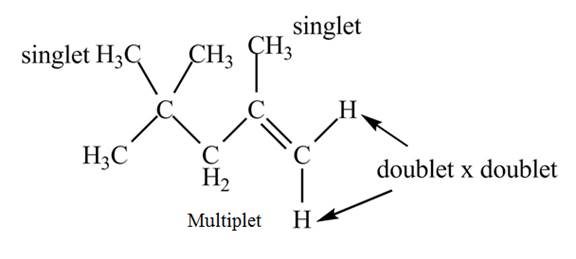
Explanation of Solution
The given spectrum is shown below.
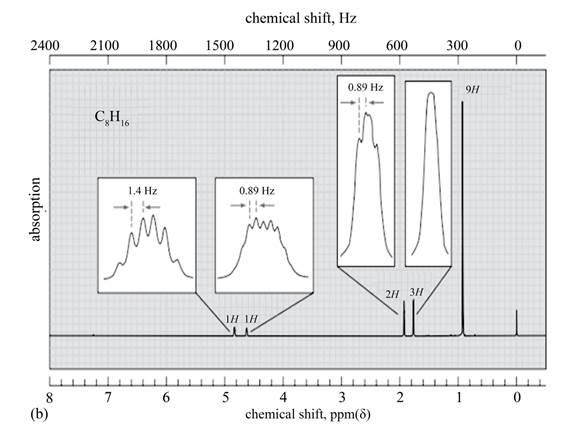
Figure 6
It is given that the
The
Figure 7
There are five signals in the compound. The two signal at
Therefore, the structure of the compound corresponding to NMR data is shown below.

Figure 8
The structure of the compound having molecular formula
(f)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
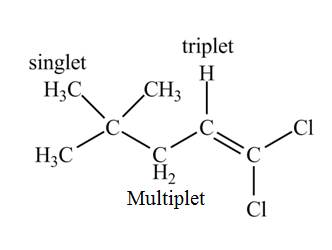
Explanation of Solution
There are three signals in the compound. The signal at
Therefore, the structure of the compound corresponds to NMR data as shown below.

Figure 9
The structure of the compound having molecular formula
(g)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
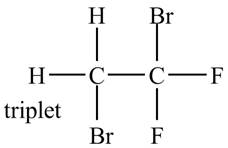
Explanation of Solution
There is only one signal in the compound for two hydrogens that means both are equivalent. The triplet signal is given by the two protons that means the splitting of the signal is done by two equivalent fluorine groups.
Therefore, the structure of the compound corresponding to NMR data is shown below.

Figure 10
The structure of the compound having molecular formula
(h)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
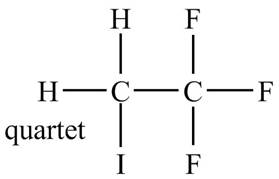
Explanation of Solution
There is only one signal in the compound for two hydrogens that means both are equivalent. The quartet signal is given by the two protons that means the splitting of the signal is done by three equivalent fluorine groups.
Therefore, the structure of the compound corresponding to NMR data is shown below.

Figure 11
The structure of the compound having molecular formula
(i)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
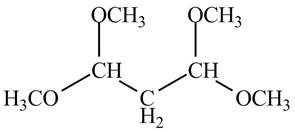
Explanation of Solution
There are three signals in the compound and total hydrogens are sixteen. The relative intergral tells about the ratio of number of hydrogen in each signal. The relative integral is
Therefore, the structure of the compound corresponding to NMR data is shown below.

Figure 12
The structure of the compound having molecular formula
(j)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
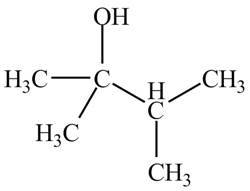
Explanation of Solution
There are three signals in the compound and total hydrogens are fourteen. The doublet signal at
The signal at
Therefore, the structure of the compound corresponding to NMR data is shown below.
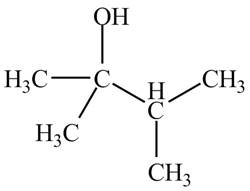
Figure 13
The structure of the compound having molecular formula
Want to see more full solutions like this?
Chapter 13 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Propose a structural formula for the analgesic phenacetin, molecular formula C10H13NO2, based on its 1H-NMR spectrum.arrow_forwardThe 1H-NMR spectrum of compound B,C7H14O , consists of the following signals: δ 0.9 (t, 6H), 1.6 (sextet, 4H), and 2.4 (t, 4H). Draw the structural formula of compound B.arrow_forwardDraw the structure of a compound with the formula C5H10O2 (along with the reasons of choosing it) which, upon analysis, gave key peaks in an infrared spectrum at 3450 cm-1 and 1713 cm-1, as well as the following 1H-NMR spectrum.arrow_forward
- Determine the following for the compound C6H15N a.) degrees of unsaturation b.) Assign the principal IR absorption bands above 1500 c.) draw the structure of the compound d.) label the protons on your structure with letters and assign them to peaks on the NMR spectrum.arrow_forwardCalculate the IHD of C7H6XNO and explain (elucidate) the structure using the 1H and 13C NMR data.arrow_forwardGive the structure for two isomers of molecular formula C4H10O which are consistent with the ^1H-NMR spectra shown below.arrow_forward
- How could 1H NMR spectroscopy be used to distinguish betweencompounds X and Y?arrow_forwardAn aromatic compound K, whose molecular formula is C8H11N, is examined in the laboratory to elucidate its structure. The following observations were made: A) Compound K is soluble in dilute hydrochloric acid but insoluble in sodium hydroxide solution. B) Treatment of compound K with excess potassium hydroxide and benzenesulfonyl chloride, C(6)H(5)SO(2)Cl, results in the formation of a heterogeneous mixture. The NMR spectrum of compound K is shown below. C) Compound K when treated with acetic anhydride[CH3-C(O)-O-C(O)-CH3], gives compound L, whose molecular formula is C(10)H(13)ON. Compound L is insoluble in dilute acid or dilute base at room temperature, heating compound L in dilute acid or base, however, regenerates compound K. D) When compound L is heated with a mixture of concentrated nitric acid and sulfuric acid, a single product, compound M, with the molecular formula C(10)H(12)O(3)N(2) is formed in excellent yields. On the basis of these observations draw the structures of…arrow_forwardPropose a structure for the molecule with the formula of C5H10O from the H NMR, C NMR, IR and mass spectrometry spectrums.arrow_forward
- The following is the predicted 1H-NMR spectrum for an unknown compound with molecular formula C6H14O . This compound is a liquid at room temperature, is slightly soluble in water, and reacts with sodium metal with the evolution of a gas.arrow_forwardCompound 1 has molecular formula C7H15Cl. It shows two signals in the 1H-NMR spectrum, one at 1.08 ppm and one at 1.59 ppm. The relative integrals of these two signals are 3 and 2, respectively. Propose structures for compound 1, explaining how you reach your conclusion.arrow_forwardThe 1H-NMR spectrum of compound R, C6H14O, consists of two signals: d 1.1 (doublet) and d 3.6 (septet) in the ratio 6:1. Propose a structural formula for compound R consistent with this informationarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
