
(a)
Interpretation:
The bubble point temperature for any one of the given binary systems in table
Concept Introduction:
Antoine equation is used to determine the vapor pressure of any substance at the given temperature by the equation:
Here,
Equation
to be used for Modified Raoult’s law is:
The Bubble point pressure for a binary system in vapor/liquid equilibrium is defined as the pressure where first bubble of vapor appears which is in equilibrium with the liquid present in the system. The equation which defines this pressure at this point is:
NRTL equations to be used are:
Here, the parameters
are calculated by the formula:
And,
are calculated by the formula:
Where,
(a)
Answer to Problem 13.50P
The bubble point temperature for
using NRTL equation is:
Explanation of Solution
Given information:
The pressure at which the bubble point temperature is to be calculated is
NRTL equation parameters are given in Table 13.10 as shown below:
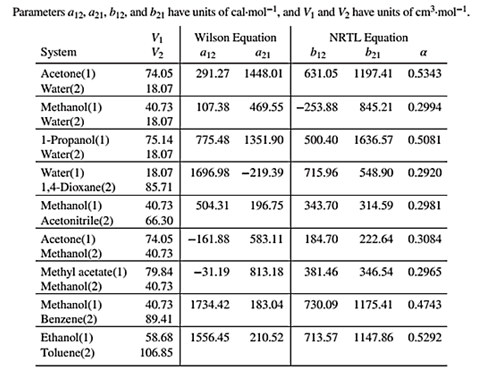
The binary system for which the bubble point temperature will be calculated is
. The liquid phase composition is:
From table B.2 of appendix B, the Antoine equation constants for
are:
Now, use equation (1) to calculate the vapor pressure of
as:
From table
to be used in NRTL equation are:
The value of universal gas constant to be used is,
Now, use equation (5) to calculate the values of
as:
Use equation (6) to calculate the values of
as:
Now, use these values of
using equations set (4) as:
Now, use the Modified Raoult’s law equation to calculate the pressure at the given value of
using the below mentioned formula as:
At
Make an initial guess for
using the preceding equations as:
(b)
Interpretation:
The dew point temperature for any one of the given binary systems in table
Concept Introduction:
Equation
to be used for Modified Raoult’s law is:
NRTL equations to be used are:
Here, the parameters
are calculated by the formula:
And,
are calculated by the formula:
Where,
The Dew point pressure for a binary system in vapor/liquid equilibrium is defined as the pressure where first drop of liquid appears which is in equilibrium with the vapor present in the system at a particular temperature. The equation that defines this pressure at this point is:
(b)
Answer to Problem 13.50P
The dew point temperature for
using NRTL equation is:
Explanation of Solution
Given information:
The pressure at which the bubble point temperature is to be calculated is
NRTL equation parameters are given in Table 13.10 as shown below:

Use the values of
as calculated in part (a) as:
The vapor phase composition is:
From table
to be used in NRTL equation are:
The value of universal gas constant to be used is,
Now, use equation (5) to calculate the values of
as:
Use equation (6) to calculate the values of
as:
Now, use these values of
using equations set (4) as:
Now, use the Modified Raoult’s law equation to calculate the pressure at the guessed value of
using the below mentioned formula as:
Make an initial guess for
as:
(c)
Interpretation:
Concept Introduction:
Equation
to be used for Modified Raoult’s law is:
The Bubble point pressure for a binary system in vapor/liquid equilibrium is defined as the pressure where first bubble of vapor appears which is in equilibrium with the liquid present in the system. The equation which defines this pressure at this point is:
The Dew point pressure for a binary system in vapor/liquid equilibrium is defined as the pressure where first drop of liquid appears which is in equilibrium with the vapor present in the system at a particular temperature. The equation that defines this pressure at this point is:
The equation for equilibrium ratio,
also known as K-value is:
Here,
The equations for flash calculations to be used are:
Here,
In terms of
is:
Here,
(c)
Answer to Problem 13.50P
The result of the
flash calculations is:
Explanation of Solution
Given information:
The flash pressure at which the
The condition for the flash temperature for this system is,
NRTL equation parameters are given in Table 13.10 as shown below:

Use the values of
as calculated in part (a) as:
To perform
To calculate bubble point temperature, let
Since, the given conditions are same as in part (a), the calculated value of
as in part (a) is:
To calculate dew point temperature, let
Since, the given conditions are same as in part (a), the calculated value of
as in part (a) is:
From the given condition of the flash temperature, it is calculated as:
Use this temperature to get the values of
as:
Now, calculate the values of
as:
Use equation (6) to calculate the values of
as:
Now, use these values of
using equations set (4) as:
Now, using the modified Raoult’s law, calculate the values of equilibrium ratio of component 1 and 2 using equations (2) and (8) as:
Now, use equation (10) and write it for both the component, 1 and 2 as shown below:
Since,
as:
Now, use equation (9) to calculate the value of
as:
Also, use the calculated value of
Using these values and the calculated values of
by equation (8) as:
Again, substitute these calculated values of
flash calculations are:
(d)
Interpretation:
The values of the azeotropic temperature and composition of the system is to be calculated if it exists for the given binary system.
Concept Introduction:
Antoine equation is used to determine the vapor pressure of any substance at the given temperature by the equation:
Here,
Equation
to be used for Modified Raoult’s law is:
NRTL equations to be used are:
Here, the parameters
are calculated by the formula:
And,
are calculated by the formula:
Where,
Relative volatility is defined by,
When
At the azeotropic point,
(d)
Answer to Problem 13.50P
The azeotropic values of temperature and composition for the binary system is calculated as:
Explanation of Solution
Given information:
The pressure at which the azeotrope of the system may exists is
NRTL equation parameters are given in Table 13.10 as shown below:

Use the given value of
as:
From table
to be used in NRTL equation are:
The value of universal gas constant to be used is,
Now, use equation (5) to calculate the values of
as:
Use equation (6) to calculate the values of
as:
Now, use these values of
Calculate
using equation (1) as:
Using equation (12) along with the modified Raoult’s law, calculate the value of relative volatility at
as:
For
using equations set (4) as:
Using equation (12) along with the modified Raoult’s law, calculate the value of relative volatility at
as:
Since
To calculate the azeotropic pressure, consider the condition
Consider the following set of equations in the given order as:
Now, use the values of
as:
Want to see more full solutions like this?
Chapter 13 Solutions
INTRO. TO CHEM.ENG.THERM. W/ ACCESS >IC
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





