
Chemistry: An Atoms First Approach
2nd Edition
ISBN: 9781305079243
Author: Steven S. Zumdahl, Susan A. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 164CWP
Consider a 0.60-M solution of HC3H5O3, lactic acid (Ka = 1.4 × 10−4).
a. Which of the following are major species in the solution?
i. HC3H5O3
ii. C3H5O3−
iii. H+
iv. H2O
v. OH−
b. Complete the following ICE table in terms of x, the amount (mol/L) of lactic acid that dissociates to reach equilibrium.
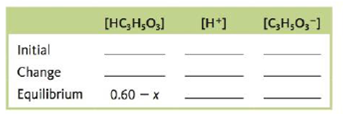
c. What is the equilibrium concentration for C3H3O3−?
d. Calculate the pH of the solution.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Calculate the concentration of the carbonate ion in a 0.010M solution of carbon dioxide in water. If all the carbonate ions in the solution come from the dissociation of HCO3^- what percentage of the Hydrogen ions in the solution are a result of this dissociation?
Part B. When acid is added to a solution of sodium hydrogen carbonate a vigorous bubbling occurs. How is this reaction related to the existence of carbonic acid molecules in aqueous solution?
Determine the molar concentration of each ion present in the solutions that result from each of the following mixtures:(Disregard the concentration of H+ and OH- from water and assume that volumes are additive.)
(a) 47.9 mL of 0.85 M HCl and 77.6 mL of 1.51 M HCl
M H+=
M Cl-=
(b) 125 mL of 0.63 M CaCl2 and 125 mL of 0.22 M CaCl2
M Ca2+=
M Cl-=
(c) 31.0 mL of 0.333 M NaOH and 22.7 mL of 0.241 M HCl
M Na+=
M Cl-=
M H+=
M OH-=
(d) 10.4 mL of 0.602 M H2SO4 and 21.5 mL of 0.151 M NaOH
M Na+=
M SO42-=
M H+=
M OH-=
using the information provided answer the following questions
1.) mass of sodium acetate trihydrate needed to prepare 100mal of a 0.100M sodium acetate solution (g)
2.) moles of sodium citrate in one bathbomb (mol)
3.) find the molarity of sodium citrate solution when one bath Bonn is dissolved in the bathtub (mol/L or M)
4.) find the pH of one bathbomb dissolved in the bathtub (assume citrate determines the pH)
5.) calculate the pH of the acetic acid/acetate Buffer prepared in part 2
Chapter 13 Solutions
Chemistry: An Atoms First Approach
Ch. 13 - Define each of the following: a. Arrhenius acid b....Ch. 13 - Define or illustrate the meaning of the following...Ch. 13 - Prob. 3RQCh. 13 - How is acid strength related to the value of Ka?...Ch. 13 - Two strategies are followed when solving for the...Ch. 13 - Prob. 6RQCh. 13 - Prob. 7RQCh. 13 - For conjugate acidbase pairs, how are Ka and Kb...Ch. 13 - What is a salt? List some anions that behave as...Ch. 13 - For oxyacids, how does acid strength depend on a....
Ch. 13 - Prob. 1ALQCh. 13 - Differentiate between the terms strength and...Ch. 13 - Sketch two graphs: (a) percent dissociation for...Ch. 13 - Prob. 4ALQCh. 13 - Prob. 5ALQCh. 13 - Prob. 6ALQCh. 13 - Prob. 7ALQCh. 13 - Prob. 8ALQCh. 13 - Consider a solution formed by mixing 100.0 mL of...Ch. 13 - Prob. 10ALQCh. 13 - Prob. 11ALQCh. 13 - Prob. 12ALQCh. 13 - What is meant by pH? True or false: A strong acid...Ch. 13 - Prob. 14ALQCh. 13 - Prob. 15ALQCh. 13 - Prob. 16ALQCh. 13 - Prob. 17ALQCh. 13 - The salt BX, when dissolved in water, produces an...Ch. 13 - Anions containing hydrogen (for example, HCO3 and...Ch. 13 - Prob. 20QCh. 13 - Prob. 21QCh. 13 - Prob. 22QCh. 13 - Prob. 23QCh. 13 - Prob. 24QCh. 13 - Prob. 25QCh. 13 - The following are representations of acidbase...Ch. 13 - Prob. 27QCh. 13 - Prob. 28QCh. 13 - Prob. 29QCh. 13 - Prob. 30QCh. 13 - Prob. 31QCh. 13 - Prob. 32QCh. 13 - Prob. 33QCh. 13 - Prob. 34QCh. 13 - Write balanced equations that describe the...Ch. 13 - Write the dissociation reaction and the...Ch. 13 - Prob. 37ECh. 13 - For each of the following aqueous reactions,...Ch. 13 - Classify each of the following as a strong acid or...Ch. 13 - Consider the following illustrations: Which beaker...Ch. 13 - Use Table 13-2 to order the following from the...Ch. 13 - Prob. 42ECh. 13 - Prob. 43ECh. 13 - Prob. 44ECh. 13 - Prob. 45ECh. 13 - Prob. 46ECh. 13 - Values of Kw as a function of temperature are as...Ch. 13 - At 40.C the value of Kw is 2.92 1014. a....Ch. 13 - Calculate the pH and pOH of the solutions in...Ch. 13 - Calculate [H+] and [OH] for each solution at 25C....Ch. 13 - Prob. 51ECh. 13 - Fill in the missing information in the following...Ch. 13 - The pH of a sample of gastric juice in a persons...Ch. 13 - The pOH of a sample of baking soda dissolved in...Ch. 13 - What are the major species present in 0.250 M...Ch. 13 - A solution is prepared by adding 50.0 mL of 0.050...Ch. 13 - Calculate the pH of each of the following...Ch. 13 - Calculate the pH of each of the following...Ch. 13 - Calculate the concentration of an aqueous HI...Ch. 13 - Prob. 60ECh. 13 - Prob. 61ECh. 13 - A solution is prepared by adding 50.0 mL...Ch. 13 - Prob. 63ECh. 13 - Prob. 64ECh. 13 - Calculate the concentration of all species present...Ch. 13 - Calculate the percent dissociation for a 0.22-M...Ch. 13 - For propanoic acid (HC3H5O2, Ka = 1.3 105),...Ch. 13 - A solution is prepared by dissolving 0.56 g...Ch. 13 - Monochloroacetic acid, HC2H2ClO2, is a skin...Ch. 13 - A typical aspirin tablet contains 325 mg...Ch. 13 - Calculate the pH of a solution that contains 1.0 M...Ch. 13 - Prob. 72ECh. 13 - Calculate the percent dissociation of the acid in...Ch. 13 - Prob. 74ECh. 13 - A 0.15-M solution of a weak acid is 3.0%...Ch. 13 - An acid HX is 25% dissociated in water. If the...Ch. 13 - Trichloroacetic acid (CCl3CO2H) is a corrosive...Ch. 13 - The pH of a 0.063-M solution of hypobromous acid...Ch. 13 - A solution of formic acid (HCOOH, Ka = 1.8 104)...Ch. 13 - Prob. 80ECh. 13 - Prob. 81ECh. 13 - You have 100.0 g saccharin, a sugar substitute,...Ch. 13 - Write the reaction and the corresponding Kb...Ch. 13 - Write the reaction and the corresponding Kb...Ch. 13 - Prob. 85ECh. 13 - Use Table 13-3 to help order the following acids...Ch. 13 - Use Table 13-3 to help answer the following...Ch. 13 - Prob. 88ECh. 13 - Calculate the pH of the following solutions. a....Ch. 13 - Calculate [OH], pOH, and pH for each of the...Ch. 13 - Prob. 91ECh. 13 - Prob. 92ECh. 13 - What mass of KOH is necessary to prepare 800.0 mL...Ch. 13 - Calculate the concentration of an aqueous Sr(OH)2...Ch. 13 - Prob. 95ECh. 13 - For the reaction of hydrazine (N2H4) in water,...Ch. 13 - Calculate [OH], [H+], and the pH of 0.20 M...Ch. 13 - Calculate [OH], [H+], and the pH of 0.40 M...Ch. 13 - Calculate the pH of a 0.20-M C2H5NH2 solution (Kb...Ch. 13 - Prob. 100ECh. 13 - What is the percent ionization in each of the...Ch. 13 - Prob. 102ECh. 13 - The pH of a 0.016-M aqueous solution of...Ch. 13 - Calculate the mass of HONH2 required to dissolve...Ch. 13 - Prob. 105ECh. 13 - Prob. 106ECh. 13 - Prob. 107ECh. 13 - Arsenic acid (H3AsO4) is a triprotic acid with Ka1...Ch. 13 - Prob. 109ECh. 13 - Calculate [CO32] in a 0.010-M solution of CO2 in...Ch. 13 - Prob. 111ECh. 13 - Calculate the pH of a 5.0 103-M solution of...Ch. 13 - Arrange the following 0.10 M solutions in order of...Ch. 13 - Prob. 114ECh. 13 - Prob. 115ECh. 13 - The Kb values for ammonia and methylamine are 1.8 ...Ch. 13 - Determine [OH], [H+], and the pH of each of the...Ch. 13 - Calculate the concentrations of all species...Ch. 13 - Prob. 119ECh. 13 - Prob. 120ECh. 13 - Prob. 121ECh. 13 - Papaverine hydrochloride (abbreviated papH+Cl;...Ch. 13 - An unknown salt is either NaCN, NaC2H3O2, NaF,...Ch. 13 - Prob. 124ECh. 13 - A 0.050-M solution of the salt NaB has a pH of...Ch. 13 - Prob. 126ECh. 13 - Prob. 127ECh. 13 - Prob. 128ECh. 13 - Are solutions of the following salts acidic,...Ch. 13 - Prob. 130ECh. 13 - Prob. 131ECh. 13 - Prob. 132ECh. 13 - Place the species in each of the following groups...Ch. 13 - Prob. 134ECh. 13 - Will the following oxides give acidic, basic, or...Ch. 13 - Prob. 136ECh. 13 - Prob. 137ECh. 13 - Prob. 138ECh. 13 - Prob. 139ECh. 13 - Zinc hydroxide is an amphoteric substance. Write...Ch. 13 - Prob. 141ECh. 13 - Prob. 142ECh. 13 - Prob. 143AECh. 13 - Prob. 144AECh. 13 - A solution is tested for pH and conductivity as...Ch. 13 - The pH of human blood is steady at a value of...Ch. 13 - Prob. 147AECh. 13 - Prob. 148AECh. 13 - Prob. 149AECh. 13 - Prob. 150AECh. 13 - Acrylic acid (CH29CHCO2H) is a precursor for many...Ch. 13 - Prob. 152AECh. 13 - Prob. 153AECh. 13 - Prob. 154AECh. 13 - Prob. 155AECh. 13 - Prob. 156AECh. 13 - Prob. 157AECh. 13 - Prob. 158AECh. 13 - Prob. 159AECh. 13 - Prob. 160AECh. 13 - Prob. 161AECh. 13 - For solutions of the same concentration, as acid...Ch. 13 - Prob. 163CWPCh. 13 - Consider a 0.60-M solution of HC3H5O3, lactic acid...Ch. 13 - Prob. 165CWPCh. 13 - Prob. 166CWPCh. 13 - Consider 0.25 M solutions of the following salts:...Ch. 13 - Calculate the pH of the following solutions: a....Ch. 13 - Prob. 169CWPCh. 13 - Prob. 170CPCh. 13 - Prob. 171CPCh. 13 - Prob. 172CPCh. 13 - Prob. 173CPCh. 13 - Prob. 174CPCh. 13 - Calculate the pH of a 0.200-M solution of C5H5NHF....Ch. 13 - Determine the pH of a 0.50-M solution of NH4OCl....Ch. 13 - Prob. 177CPCh. 13 - Prob. 178CPCh. 13 - Consider 1000. mL of a 1.00 104-M solution of a...Ch. 13 - Calculate the mass of sodium hydroxide that must...Ch. 13 - Prob. 181CPCh. 13 - Prob. 182CPCh. 13 - Will 0.10 M solutions of the following salts be...Ch. 13 - Prob. 184CPCh. 13 - A 0.100-g sample of the weak acid HA (molar mass =...Ch. 13 - Prob. 186CPCh. 13 - A 2.14 g sample of sodium hypoiodite is dissolved...Ch. 13 - Isocyanic acid (HNCO) can be prepared by heating...Ch. 13 - Prob. 189IPCh. 13 - An aqueous solution contains a mixture of 0.0500 M...Ch. 13 - Prob. 191MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the same symbols as in Question 61 ( = anion, =OH) for the box below. (a) Fill in a similar box (representing one liter of the same solution) after 2 mol of H+ (2) have been added. Indicate whether the resulting solution is an acid, base, or buffer. (b) Follow the directions of part (a) for the resulting solution after 2 mol of OH- (2 ) have been added. (c) Follow the directions of part (a) for the resulting solution after 5 mol of OH- (5 ) have been added. (Hint: Write the equation for the reaction before you draw the results.)arrow_forwardA solution is prepared from 0.150 mol of formic acid and enough water to make 0.425 L of solution. a Determine the concentrations of H3O+ and HCOO in this solution. b Determine the H3O+ concentration that would be necessary to decrease the HCOO concentration above by a factor of 10. How many milliliters of 2.00 M HCl would be required to produce this solution? Consider that the solution was made by combining the HCl, the HCOOH, and enough water to make 0.425 L of solution. c Qualitatively, how can you account for the differences in the percentage dissociation of formic acid in parts a and b of this problem?arrow_forwardStrong Acids, Weak Acids, and pH Two 0.10-mol samples of the hypothetical monoprotic acids HA(aq) and HB(aq) are used to prepare 1.0-L stock solutions of each acid. a Write the chemical reactions for these acids in water. What are the concentrations of the two acid solutions? b One of these acids is a strong acid, and one is weak. What could you measure that would tell you which acid was strong and which was weak? c Say that the HA(aq) solution has a pH of 3.7. Is this the stronger of the two acids? How did you arrive at your answer? d What is the concentration of A(aq) in the HA solution described in part c? e If HB(aq) is a strong acid, what is the hydronium-ion concentration? f In the solution of HB(aq), which of the following would you expect to be in the greatest concentration: H3O+(aq), B(aq), HB(aq), or OH(aq)? How did you decide? g In the solution of HA(aq), which of the following would you expect to be in the greatest concentration: H3O+(aq), A+(aq), HA(aq), or OH(aq)? How did you decide? h Say you add 1.0 L of pure water to a solution of HB. Would this water addition make the solution more acidic, make it less acidic, or not change the acidity of the original solution? Be sure to fully justify your answer. i You prepare a 1.0-L solution of HA. You then take a 200-mL sample of this solution and place it into a separate container. Would this 200 mL sample be more acidic, be less acidic, or have the same acidity as the original 1.0-L solution of HA(aq)? Be sure to support your answer.arrow_forward
- A solution is tested for pH and conductivity as pictured below: The solution contains one of the following substances: HCl. NaOH, NH4Cl. HCN, NH3, HF, or NaCN. If the solute concentration is about 1.0 M, what is the identity of the solute?arrow_forwardTwo strategies are also followed when solving for the pH of a base in water. What is the strategy for calculating the pH of a strong base in water? List the strong bases mentioned in the text that should be committed to memory. Why is calculating the pH of Ca(OH)2 solutions a little more difficult than calculating the pH of NaOH solutions? Most bases are weak bases. The presence of what element most commonly results in basic properties for an organic compound? What is present on this element in compounds that allows it to accept a proton? Table 13-3 and Appendix 5 of the text list Kb values for some weak bases. What strategy is used to solve for the pH of a weak base in water? What assumptions are made when solving for the pH of weak base solutions? If the 5% rule fails, how do you calculate the pH of a weak base in water?arrow_forwardConsider the following six beakers. All have 100 mL of aqueous 0.1 M solutions of the following compounds: beaker A has HI beaker B has HNO2 beaker C has NaOH beaker D has Ba(OH)2 beaker E has NH4Cl beaker F has C2H5NH2 Answer the questions below, using LT (for is less than), GT (for is greater than), EQ (for is equal to), or MI (for more Information required). (a) The pH in beaker A the pH in beaker B. (b) The pH in beaker C the pH in beaker D. (c) The % ionization in beaker A the % ionization in beaker C. (d) The pH in beaker B the pH in beaker E. (e) The pH in beaker E the pH in beaker F. (f) The pH in beaker C the pH in beaker F.arrow_forward
- Explain why a sample of pure water at 40 C is neutral even though [H3O+]=1.7107M . Kw is 2.91014 at 40 C.arrow_forwardThe following illustration displays the relative number of species when an acid, HA, is added to water. a. Is HA a weak or strong acid? How can you tell? b. Using the relative numbers given in the illustration, determine the value for Ka and the percent dissociation of the acid. Assume the initial acid concentration is 0.20 M.arrow_forwardWhat is the freezing point of 0.92 M aqueous acetic acid? The density of this solution is 1.008 g/cm3.arrow_forward
- One way to make vinegar (not the preferred way) is to prepare a solution of acetic acid, the sole acid component of vinegar, at the proper pH and add appropriate flavoring agents. Acetic acid (Mr 60) is a liquid at 25 °C, with a density of 1.049 g/mL. Calculate the volume that must be added to distilled water to make 1 L of simulated vinegararrow_forwardCalculate the molarity of a sodium hydroxide solution if 10.42 mL of this solution are needed to neutralize 25.00 mL of 0.2043 M oxalic acid. H2C2O4 (aq) + 2 NaOH (aq) → Na2C2O4 (aq) + 2 H2O (l) Group of answer choices 0.9803 M 1.500 M 0.1042 M 0.4902 M 0.2500 Marrow_forwardDetermine the pH of each solution. Part A 0.13 M NH4Cl (Kb(NH3)=1.76×10^−5) 0.18 M NaC2H3O2 (Ka(HC2H3O2)=1.8×10^−5) 0.19 M NaClarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY