
Concept explainers
(a)
Interpretation:
The electron-pair geometry for the molecules,
Concept introduction:
The electron pairs in Lewis diagrams repel each other in real molecule and thus they distribute themselves in positions around the central atoms that are as far away from one another. This arrangement of electron pairs is called electron-pair geometry. The electron pairs may be shared in covalent bond, or they may be lone pairs.
Answer to Problem 22E
The Lewis diagrams for
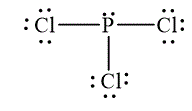 and
and 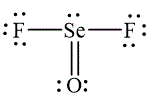
The wedge-and-dash diagrams for
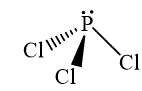 and
and 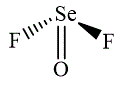
The electron pair geometry for both molecules is tetrahedral.
Explanation of Solution
To write the Lewis diagram for a compound first the number of valence electrons is to be calculated. In the molecule,
Similarly, in the molecule,
The atom which is least electronegative is the central atom. In
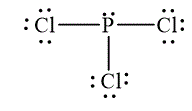
Figure 1
In
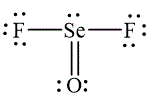
Figure 2
The electron-pair geometry depends on the number of electron pairs around the central atoms. In both the molecules
The wedge-and-dash diagram for the molecules
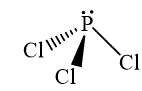
Figure 3
The wedge-and-dash diagram for the molecules

Figure 4
The Lewis and wedge-and-dash diagrams for
(b)
Interpretation:
The molecular geometry prdicted by the valence shell electron-pair repulsion theory for the molecules
Concept introduction:
Molecular geometry is the precise term that is used to describe the shape of molecules and arrangement of atoms around the central atom. The molecular geometry of a molecule is predicted by valence shell electron-pair repulsion theory or in short VSEPR theory. VSEPR theory applies to substances in which a second period element is bonded to two, three, four, or other atoms.
Answer to Problem 22E
The Lewis diagrams for
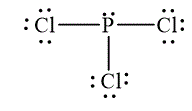 and
and 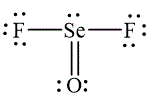
The wedge-and-dash diagrams for
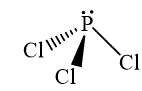 and
and 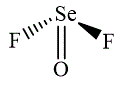
The molecular geometry for both molecules is trygonal pyramidal.
Explanation of Solution
To write the Lewis diagram for a compound first the number of valence electrons is to be calculated. In the molecule,
Similarly, in the molecule,
The atom which is least electronegative is the central atom. In
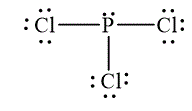
Figure 1
In
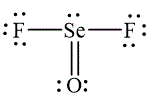
Figure 2
The molecular geometry depends on the number of electron pairs around the central atoms and the number of lone pairs present on the central atom. In the both of the molecules,
The wedge-and-dash diagram for the molecules
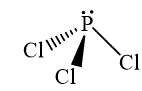
Figure 3
The wedge-and-dash diagram for the molecules
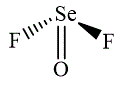
Figure 4
The Lewis and wedge-and-dash diagrams for
Want to see more full solutions like this?
Chapter 13 Solutions
Introductory Chemistry: An Active Learning Approach
- According to VSEPR theory, what determines the geometry of a molecule?arrow_forward1.) Summarize the octet rule. Atoms have a tendency to lose, gain, or share electrons such that their (______________) valence shell or core shell results in an octet of electrons.In other words, atoms have a tendency to lose, gain, or share electrons so as to achieve the (_______________) stable or unstable electronic configuration of the (___________) inert metal or noble gas or reactive gas or reactive metal . 2.) Using the VSEPR model, identify the molecular geometry of nitrogen tribromide, NBr3, based on the number of electron domains. Circle the correct answer! Choose only 1. Linear trigonal planar bent tetrahedral trigonal pyramidal trigonal bipyramidal seesaw T-shaped Octahedral square pyramidal square planar 3.) What is the difference between sigma and pi bonds? You only have one attempt at this question. Circle the correct answer! Choose only 1. sigma bonds are the overlap of s-orbitals sigma bonds are the overlap of both s- and p-orbitals pi bonds are the…arrow_forwardDraw the shapes of the following molecules and ions in 3-dimension. Show clearly any lone pairs of electrons on the central atom, state the number of bond pairs and lone pairs of electrons on the central atom and name the shape of the molecule or ion. (a) PH4+, phosphonium ion (b) PBr5, phosphorus pentabromide (c) H3O+, oxonium ion Provide everything stated in the instructions for each compound.arrow_forward
- Step 1 – Write the Lewis structure from the molecular formula.Step 2 – Assign an electron-group arrangement by counting all electron groups (bonding plus nonbonding) around the central atom (or around each centralatom, if more than one central atom in structure).Step 3 – Predict the ideal bond angle from the electron-group arrangement and the effect of any deviation caused by lone pairs or double bonds.Step 4 – Name the molecular shape by counting bonding groups and nonbonding groups separately.Step 5 – Predict whether the molecule is polar or nonpolarStep 6 – Describe the hybridization around the central atom and identify the total number of σ and π bonds in the structurearrow_forwardDraw the shapes of the following molecules and ions in 3-dimension. Show clearly any lone pairs of electrons on the central atom, state the number of bond pairs and lone pairs of electrons on the central atom and name the shape of the molecule or ion. (a) AIH4-, aluminium hydride ion (b) CH3-, methyl carbanion (c) POCl3, phosphorus oxychloride Provide everything stated in the instructions for each compound.arrow_forwardH20Sketch the proper Lewis structure for this substance. Be sure to follow octet/duet rules for each atom and use the totalnumber of valence electrons available. Use your drawing to answer the following questions.Count the number of each type of electron domain surrounding the central atom. Do not leave any blank empty. enter O if itis unused.Lone pairs: Single bonds: Double bonds: Triple bonds: Count the total number of electron domains surrounding the central atom. Enter the name corresponding to the electron domain geometry. Options are: linear, trigonal planar, tetrahedral.arrow_forward
- Germanium chloride, GeCl2, has only two atoms surrounding the central germanium atom. Why then is the germanium chloride molecule bent? Group of answer choices There is a covalent bond between the two chlorine atoms. Lone pairs of electrons on the chlorine atoms push it to this orientation. It is bent only periodically as it swings between both bent and linear shapes. A lone pair of electrons on germanium pushes it to this orientation.arrow_forwardFind the total number of valence electrons, lewis structure, name of electron and molecular geometry, and 3D diagram for SEI2 and SbBr3.arrow_forwardPlease count total valence electrons, have lewis structures, resonance structures when applicable, and name the molecular geometry for each central atom.arrow_forward
- Your assigneed ion is : AlCl4- please give a brief description that includes: (c) the arrangement (or shape) of the electron grouping (includes lone pairs), (e) actual molecular geometry (may be the same or different than answer (c), Please note that your individual assignment may be for an ion (don't forget about charges) or a neutral atom.arrow_forwardThis compound has the formula C6H5CH3ClCH=CHCHO Each intersection of adjoining lines is a carbon atom. =C- or -C- a sketch of this molecule showing every hydrogen atom needed to complete octets and any lone pairs of electrons that are needed to complete the oxygen and chlorine atom octets Write by the following atoms the VSEPR geometry on the drawing as linear, bent, trigonal planar, bent, trigonal pyramidal or tetrahedral for the atoms: The carbon on the right hand side directly bonded to Oxygen The carbon that is CH3 on the left side The carbons in the ring (all the same)arrow_forwardWrite answers to the following questions for these compounds: SO2 (sulfur in the middle with the two oxygens attached to it) C2H2 (carbons attached to each other with one hydrogen attached to each carbon CO2 (carbon in the middle with two oxygens attached to it). 1. determine the number of valence electrons for each compound 2. draw a Lewis structure and include non-bonding electrons if necessary 3. write the electron group geometry and molecular geometry terms 4. determine whether or not the compound is polar or non-polararrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

