
(a)
Interpretation:
The electron-pair geometry for each carbon and nitrogen atoms in the molecule
Concept introduction:
The electron pairs in Lewis diagrams repel each other in real molecule and thus they distribute themselves in positions around the central atoms which are far away from one another. This arrangement of electron pairs is called electron-pair geometry. The electron pairs may be shared in covalent bond, or they may be lone pairs.
Answer to Problem 28E
The Lewis diagram for the molecule
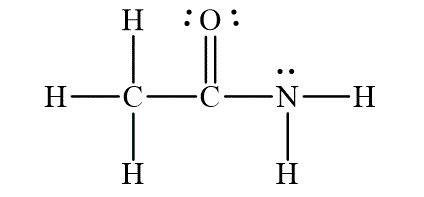
The electron pair geometry for the carbon
Explanation of Solution
To write the Lewis diagram for a compound first the number of valence electrons is to be calculated. In the molecule
In the molecule

Figure 1
The electron-pair geometry depends on the number of electron pairs around the central atoms. In the molecule
The Lewis diagram for the molecule
(b)
Interpretation:
The molecular geometry predicted by the valence shell electron-pair repulsion theory for each carbon and nitrogen atoms in the molecule
Concept introduction:
Molecular geometry is the precise term that is used to describe the shape of molecules and arrangement of atoms around the central atom. The molecular geometry of a molecule is predicted by valence shell electron-pair repulsion theory or in short VSEPR theory. VSEPR theory applies to substances in which a second period element is bonded to two, three, four, or other atoms.
Answer to Problem 28E
The Lewis diagram for the molecule

The molecular geometry for the carbon
Explanation of Solution
To write the Lewis diagram for a compound first the number of valence electrons is to be calculated. In the molecule
In the molecule

Figure 1
The molecular geometry depends on the number of electron pairs as well as number of lone-pair electrons on the central atoms. In the molecule
The Lewis diagram for the molecule
Want to see more full solutions like this?
Chapter 13 Solutions
Introductory Chemistry: An Active Learning Approach
- Classify each of the following statements as true or false: a Molecular geometry around an atom may or may not be the same as electron-pair geometry around the atom. b Electron pair geometry is the direct effect of molecular geometry. c If the geometry of a molecule is linear, the molecule must have at least one double bond. d A molecule with a double bond cannot have trigonal pyramidal geometry around the double bonded atom. e A CO2 molecule is linear, but an SO2 molecule is bent. f A molecule is polar if it contains polar bonds. g A molecule with a central atom that has one lone pair of electrons is always polar. h A molecule with a central atom that has two lone pairs and two bonded pairs of electrons is always polar. i Carbon atoms normally form four bonds. j Hydrogen atoms never form double bonds.arrow_forwardFor each of the following ions/molecules, state the number of bond pairs state the number of lone pairs state the bond angle(s) state, or draw, the shapearrow_forwardComplete Table 3 (Remember that the values pertain to the Central atoms only. In this table, you will have more than one central atom so report more than one value, ie. 3 / 3) Table 3 Molecules with Multiple Central Atoms Molecule C2H4 H2O2. CH3OH. CH3NH2 Number of Bond Pairs / / / / Number of Lone Pairs / / / / Number of Electron Domains / / / / Molecular Geometry (Shape) / / / / Bond Angle / / / / Polar or Non-Polar Nonpolar Polar Polar Polararrow_forward
- Answer the following questions about the Lewis structure for the organic molecule CH3CH2CH2OH (see examples in Chapter 10) There are Blank 1. Fill in the blank, read surrounding text. valence electrons. The molecule has Blank 2. Fill in the blank, read surrounding text. single bonds, Blank 3. Fill in the blank, read surrounding text. double bonds, and Blank 4. Fill in the blank, read surrounding text. triple bonds. There are Blank 5. Fill in the blank, read surrounding text. central C atoms, each has Blank 6. Fill in the blank, read surrounding text. REDs, and the shape is Blank 7. Fill in the blank, read surrounding text. . The central O has Blank 8. Fill in the blank, read surrounding text. REDs and the shape is Blank 9. Fill in the blank, read surrounding text. . The bond angles at every central atom is Blank 10. Fill in the blank, read surrounding text. . Since there are Blank 11. Fill in the blank, read surrounding text. polar covalent bonds that do not cancel…arrow_forwardDetermine the molecular geometry around each carbon atom in propylene, C3H6. Sketch a wedge-and-dash diagram of the molecule.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co




