
Concept explainers
(a)
Interpretation:
The resulting alcohol should be identified after reacting following alkene with

Concept Introduction:
Reaction of an alkene with
The hydration reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 56P
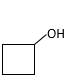
Explanation of Solution
Unsaturated (
(b)
Interpretation:
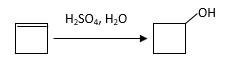
The resulting alcohol should be identified after reacting following alkene with
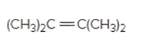
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Reaction of an alkene with
Hydration reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 56P
Explanation of Solution
Unsaturated (
(c)
Interpretation:
The resulting alcohol should be identified after reacting following alkene with
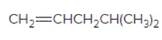
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Reaction of an alkene with
Hydration reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 56P
Explanation of Solution
Unsaturated (
Refer to the below reaction:
(d)
Interpretation:
The resulting alcohol should be identified after reacting following alkene with
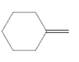
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Reaction of an alkene with
Hydration reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 56P
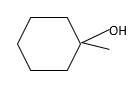
Explanation of Solution
Unsaturated (
Refer to the below reaction:
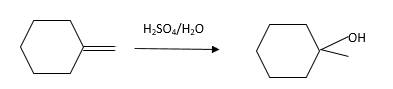
Want to see more full solutions like this?
Chapter 13 Solutions
GENERAL, ORGANIC & BIOLOGICAL CHEMISTRY
- What alcohol is formed when the compound is treated with H2 and a Pd catalyst?arrow_forwardGive the IUPAC name of the alcohol that fits each of the following descriptions. a. Moistening agent in many cosmetics b. Major ingredient in environmentally friendly antifreeze formulations c. Industrially produced from CO and H2 d. Often produced via a fermentation processarrow_forwardWhat alkenes are formed when each alcohol is dehydrated with TsOH? Label the major product when a mixture resultsarrow_forward
- What alcohol is formed when the following compound is treated with H2 and a Pd catalyst?arrow_forwardWhat products are formed when benzene is treated with each alkyl chloride and AlCl3?arrow_forwardALCOHOLS 1. WHY IS ETHANOL MORE SOLUBLE IN WATER THAN 1-HEXANOL? 2. WHAT IS DENATURED ALCOHOL? AND WHY IS ALCOHOL DENATURED? ETHER 1. WHY DOES DIETHYL ETHER HAVE MUCH LOWER BOILING POINT THAN 1-BUTANOL?arrow_forward
- What alcohol is formed when each carbonyl compound is treated with H 2 and a Pd catalyst?arrow_forwardGive the structure corresponding to each IUPAC name. 2,4 dimethyl- 2 hexanolarrow_forwardWhat product is formed when benzene is treated with each organic halide in the presence of AlCl3?arrow_forward
- What alkenes are formed when each alcohol is treated with H 2SO 4? Use the Zaitsev rule to predict the major product.arrow_forwardWhat alkenes are formed when attached alcohol is dehydrated with TsOH?Label the major product when a mixture resultsarrow_forwardGive the structure corresponding to each IUPAC name. a. 3-methyl-3-pentanold. 1,3-propanediol b. 4-methyl-2-pentanole. 3,5-dimethylcyclohexanol c. 2,4-dimethyl-2-hexanolf. 6,6-diethyl-4-nonanolarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


