
Concept explainers
(a)
Interpretation: The major product in the reaction needs to be identified.
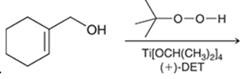
Concept introduction: The problem is based on the concept of enantioselective epoxidation. In this process, if a chiral
Here for enantioselective epoxidation of allylic alcohols where the hydroxyl group is attached to the allylic position which is next to a C-C double bond, a catalyst is developed. This catalyst contains titanium tetraisopropoxide and enantiomer of diethyl tartrate.
(b)
Interpretation: The major product in the reaction needs to be identified.
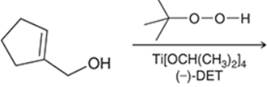
Concept introduction: The problem is based on the concept of enantioselective epoxidation. In this process, if a chiral epoxide needs to be formed, a racemic mixture of epoxide will be formed.
Here for enantioselective epoxidation of allylic alcohols where the hydroxyl group is attached to the allylic position which is next to a C-C double bond, a catalyst is developed. This catalyst contains titanium tetraisopropoxide and enantiomer of diethyl tartrate.
(c)
Interpretation; The major product in the reaction needs to be identified.
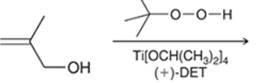
Concept introduction: The problem is based on the concept of enantioselective epoxidation. In this process, if a chiral epoxide needs to be formed, a racemic mixture of epoxide will be formed.
Here for enantioselective epoxidation of allylic alcohols where the hydroxyl group is attached to the allylic position which is next to a C-C double bond, a catalyst is developed. This catalyst contains titanium tetraisopropoxide and enantiomer of diethyl tartrate.
(d)
Interpretation: The major product in the reaction needs to be identified.
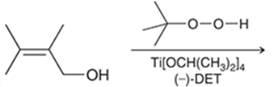
Concept introduction: The problem is based on the concept of enantioselective epoxidation. In this process, if a chiral epoxide needs to be formed, a racemic mixture of epoxide will be formed.
Here for enantioselective epoxidation of allylic alcohols where the hydroxyl group is attached to the allylic position which is next to a C-C double bond, a catalyst is developed. This catalyst contains titanium tetraisopropoxide and enantiomer of diethyl tartrate.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Predict the expected products of the following reactions, include the appropriate stereochemistry in the structure where necessary.arrow_forwardIdentify the expected major product for the following reaction:arrow_forwardPredict the product(s) and provide the mechanism for each reaction below.arrow_forward
