
Concept explainers
(a)
Interpretation:
The product on reaction of
Concept introduction:
The
Answer to Problem 14.27AP
The product on reaction of
Explanation of Solution
The reaction of
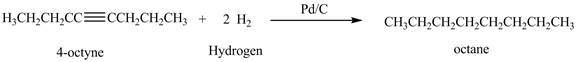
Figure 1
In the above reaction, octyne reacts with two moles of hydrogen to form octane. Octyne is an unsaturated molecule consisting of a triple bond. It reacts with hydrogen to form octane. Therefore, the product on reaction of
The product on reaction of
(b)
Interpretation:
The product on reaction of
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.27AP
The product on reaction of
Explanation of Solution
The reaction of

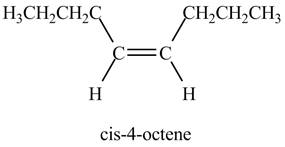
Figure 2
In the above reaction, octyne reacts with hydrogen to form octene. Octyne is an unsaturated molecule consisting of a triple bond. It reacts with hydrogen in presence of Lindlar’s catalyst to form
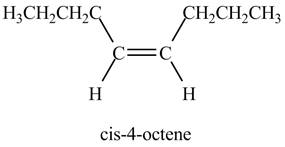
Figure 3
The product on reaction of
(c)
Interpretation:
The product on reaction of the product formed in part (b) with
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.27AP
The product on reaction of the product formed in part (b) with
Explanation of Solution
The product formed in part (b) is shown below.

Figure 4
The reaction of the product formed in part (b) with
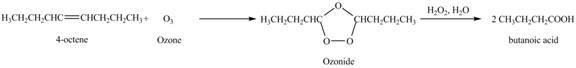
Figure 5
In the above reaction, octene a product from part (b) reacts with ozone to form ozonide. This ozonide formed hydrolysis to give two moles of butanoic acid as shown in figure 6. Therefore, the product formed on ozonolysis of octene is butanoic acid,
The product on reaction of the product formed in part (b) with
(d)
Interpretation:
The product on reaction of the
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.27AP
The product on reaction of the
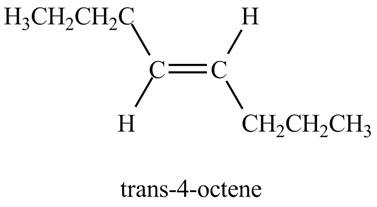
Explanation of Solution
The reaction of
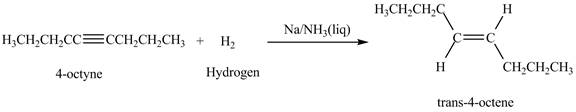
Figure 6
In the above reaction, octyne reacts with sodium in liquid ammonia to form

Figure 7
The product on reaction of the
(e)
Interpretation:
The product on reaction of
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.27AP
The product on reaction of the
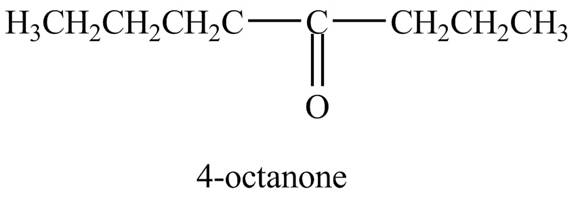
Explanation of Solution
The reaction of

Figure 8
In the above reaction, octyne reacts with
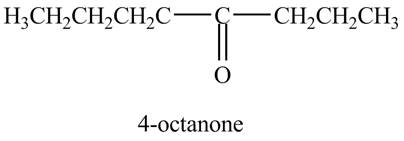
Figure 9
The product on reaction of the
(f)
Interpretation:
The product on reaction of the
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.27AP
The product formed on reaction of
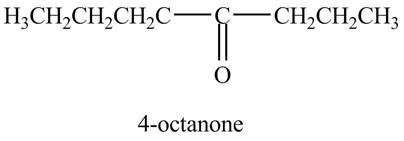
Explanation of Solution
The reaction of

Figure 10
In the above reaction, octyne reacts with boron hydride to form organoborane. Organoborane reacts with peroxide and hydroxide ion to form an enol. This enol tautomerises to form
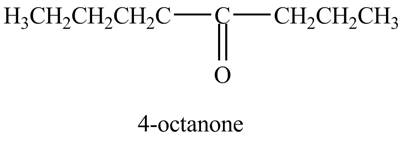
Figure 11
The product on reaction of the
Want to see more full solutions like this?
Chapter 14 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- When the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forwardOne frequently used method for preparing methyl esters is by reaction of carboxylic acids with diazomethane, CH2N2. The reaction occurs in two steps: (l) protonation of diazomethane by the carboxylic acid to yield methyldiazonium ion, CH3N2+, plus a carboxylate ion; and (2) reaction of the carboxylate ion with CH3N2+. (a) Draw two resonance structures of diazomethane, and account for step 1. (b) What kind of reaction occurs in step 2?arrow_forwardIn an advanced synthetic chemistry experiment, a researcher prepares a compound, ZY-7, by reacting a ketone (C5H10O) with hydroxylamine (NH2OH), followed by heating in the presence of an acid catalyst. The resulting compound, ZY-7, is then treated with a solution of sodium nitrite (NaNO2) and hydrochloric acid (HCl) at low temperature. Identify the class of compound that ZY-7 most likely belongs to after this series of reactions." A) Amide B) Oxime C) Nitro compound D) Diazonium salt E) Ester Don't use chatgpt please provide valuable answerarrow_forward
- Arrange the following molecules in increasing order of acidity. Base it only on their structural differences and explain how it is so. 1. HF, CH3CH2CH2OH, CH3CH2COOH 2. Ethyl amine, Ethanol, Propanearrow_forward5. Compound A, C 10H 18O, undergoes reaction with dilute H 2SO 4 at 50 °C to yield a mixture of two alkenes, C 10H 16. The major alkene B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Which of the following reactions are correct.arrow_forwardCompound A (C6H12O2) reacts with water, acid, and heatto yield compound B (C5H10O2) and compound C (CH4O).Compound B is acidic. Deduce possible structures of compounds A, B, and Carrow_forward
- Write reactions of ethyl chloride with the following reagents: a. KOH, aqueous solution b. NaCNarrow_forwardCompound A, C 10H 18O, undergoes reaction with dilute H 2SO 4 at 50 °C to yield a mixture of two alkenes, C 10H 16. The major alkene B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Which of the following reactions are correct. Can be more than one answerarrow_forwardDraw a resonance structure of the acetonitrile anion, -: CH2CN, and account for the acidity of nitriles.arrow_forward
- Hydrocarbon X has the formula C6H12.X reacts with one molar equivalent of hydrogen in the presence of a palladium catalyst to form a product having 12 primary hydrogens.Treatment of X with ozone followed by zinc in aqueous acid gives a mixture two aldehydes.What is the structure of X?arrow_forwardPropose structures for molecules that fit the following descriptions:(a) An aldehyde with the formula C5H10O(b) An ester with the formula C6H12O2(c) A compound with the formula C3H7NOS that is both anamide and a thiolarrow_forward2. An unknown hydrocarbon A with the formula C 6H 12 reacts with 1 molar equivalent of H 2 over palladium catalyst. Hydrocarbon A also reacts with OsO 4 to give diol B. When oxidized with KMnO 4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH 2CO 2H and the other fragment is a ketone. Which of the following are correctarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning




