
Concept explainers
(a)
Interpretation:
The dehydrate product formed from the following alcohol with
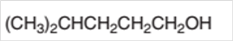
Concept Introduction:
A
In a chemical reaction, the substance which is involved in conversion is said to be a reactant, whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form
Answer to Problem 14.62P
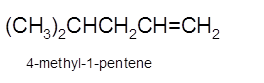
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH group from two adjacent C's
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
Hence, the dehydration of 4-methylpentanol will form 4-methyl-1-pentene molecule.
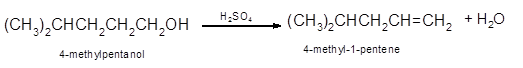
(b)
Interpretation:
The dehydrate product formed from the following alcohol with
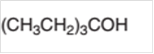
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form alkene in the presence of
Answer to Problem 14.62P
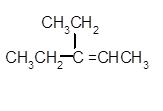
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH group from two adjacent C's
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
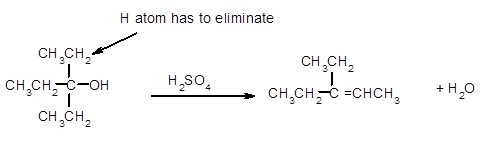
(c)
Interpretation:
The dehydrated product formed from the following alcohol with
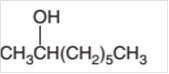
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form alkene in the presence of
Answer to Problem 14.62P
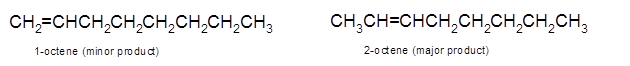
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH group from two adjacent C's
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
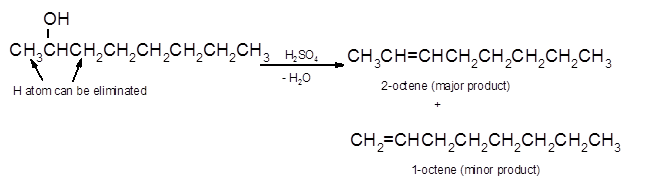
(d)
Interpretation:
The dehydrate product formed from the following alcohol with
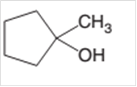
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form alkene in the presence of
Answer to Problem 14.62P
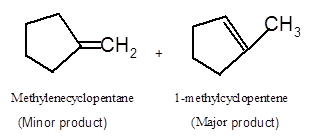
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH group from two adjacent C's
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
Want to see more full solutions like this?
Chapter 14 Solutions
GENERAL ORGANIC+BIOCHEM (LL)W/CONNECT
- 1) Draw each alcohol2) Categorize the alcohol as primary, secondary or tertiary3) Oxidize each alcohol as many times as possible4) Name the product(s) if there are any molecule: 3-Tertbutylcyclopropanolarrow_forwardWhat products are formed when each alcohol is oxidized with K2Cr2O7? a. CH3CH2CH2CH2CH2OHarrow_forwardWhat products are formed when each alcohol is oxidized with K 2Cr 2O 7? In some cases, no reaction occurs.arrow_forward
- Draw the carbonyl products formed when each alcohol is oxidized with K 2Cr 2O 7.arrow_forwardWhat product is formed when the alcohol is oxidized with K2Cr2O7? In some cases, no reaction occurs (if so, draw the given alcohol).arrow_forward1. Which alcohol has a higher boiling point?a. (i) 2-methylpropan-2-ol or (ii) butan-2-olb. (i) hexan-1-ol or (ii) 3,3-dimethylbutan-1-olarrow_forward
- Show how each alcohol or diol can be prepared from an alkene. (a) 2-Pentanol (b) 1-Pentanol (c) 2-Methyl-2-pentanol (d) 2-Methyl-2-butanol (e) 3-Pentanol (f) 3-Ethyl-3-pentanol (g) 1,2-Hexanediolarrow_forwardWhich of the following alcohols can be prepared from a Grignard reagent and ethylene oxide? A. only 1 B. only 1 and 2 C. only 1, 2 and 3 D. 1, 2, 3 and 4arrow_forwardGive a systematic (IUPAC) name for each diol. ) HO¬(CH2)8¬OHarrow_forward
- Identify the IUPAC name of the given structure. A. 2 - methylhexan-5-one B. 5 - methylhexan-2-one C. 2 - heptanone D. 5 - heptanone Identify the IUPAC name of the given structure. A. 4 - bromopentan-3-one B. 1 - bromobutan-2-one C. 2 - bromobutan-one D. None of the abovearrow_forward1. O-hydroxybenzoic acid is a major product formed with phenol and which other reactant/s I-primary alcohol II-sodium hydroxide III-water IV-carbon dioxide A.I and III B. I and IV C. II and III D. II and IVarrow_forwardDraw the reaction of butane-1,2-diol with (CH3)2COarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



