
Identify each structural formula as belonging to an
- (a) CH3CH2NH2
- (b)
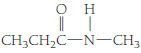
- (c)
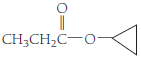
- (d)
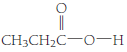
- (e)
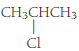
- (f)
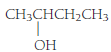
(a)
Answer to Problem 15E
Explanation of Solution
Given Info: The compound is
Explanation:
The condensed structural formula of a given compound is
The general formula of an amine is
Therefore, the compound
Conclusion:
Therefore, the compound
(b)
Answer to Problem 15E
Explanation of Solution
Given Info: The compound is
Explanation:
The condensed structural formula of a given compound is
The general formula of an amide is
Therefore, the compound
Conclusion:
Therefore, the compound
(c)
Answer to Problem 15E
Explanation of Solution
Given Info: The compound is cyclopropyl propanoate.
Explanation:
The IUPAC name of given compound is cyclopropyl propanoate. The compound contains two oxygen atoms.
The general formula for an ester is
Therefore, the compound cyclopropyl propanoate belongs to an ester.
Conclusion:
Therefore, the compound cyclopropyl propanoate belongs to an ester.
(d)
Answer to Problem 15E
Explanation of Solution
Given Info: The compound is
Explanation:
The condensed structural formula of a given compound is
The general formula of a carboxylic acid is
Therefore, the compound
Conclusion:
Therefore, the compound
(e)
Answer to Problem 15E
Explanation of Solution
Given Info: The compound is
Explanation:
The condensed structural formula of a given compound is
The general formula of an alkyl halide is
Therefore, the compound
Conclusion:
Therefore, the compound
(f)
Answer to Problem 15E
Explanation of Solution
Given Info: The compound is
Explanation:
The condensed structural formula of a given compound is
The general formula of an alcohol is
Therefore, the compound
Conclusion:
Therefore, the compound
Want to see more full solutions like this?
Chapter 14 Solutions
An Introduction To Physical Science College Of The Canyons 14th Edition And Real World Science Physics And Chemistry Applications Lab Manual
Additional Science Textbook Solutions
Loose Leaf For Explorations: Introduction To Astronomy
The Physical Universe
University Physics Volume 1
Schaum's Outline of College Physics, Twelfth Edition (Schaum's Outlines)
Tutorials in Introductory Physics
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning


