
Concept explainers
Interpretation:
The infrared spectra for carvone and limonene needs to be interpreted. Also, the proton and carbon-13 NMR spectra of carvone needs to be explained.
Concept Introduction:
In the IR spectroscopy, the IR region of the
Nuclear Magnetic Resonance (NMR) spectroscopy is a logical chemistry method used in quality control.
It studies for defining the material and purity of a sample and their molecular structure. It explains magnetic energy levels undergoing the resonance transition when the atomic nuclei are exposed to an external magnetic field and an electromagnetic radiation is applied with the specific frequency. The NMR spectrum is obtained by detecting the absorption signals.
Explanation of Solution
The structure of limonene is represented as follows:
Limonene will have C-H stretching at 3000 cm-1 and C=C stretching at 1645 cm-1
Now, the structure of Carvone is represented as follows:
Carvone will have C=C stretching at 1645 cm-1 and a, β-unsaturated
The structures of Carvone are given below along with the NMR interpretation (both 1H and 13C).
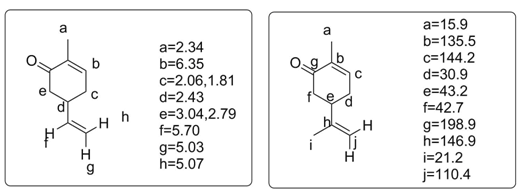
Carvone NMR (1H and 13C)
In the NMR of Carvone two methyl peaks are there along with vinylic protons. There are few CH2 protons. In 13-C there are a, β-unsaturated ketone peak along with C=C peaks. Two methyl peaks and CH2 peaks.
In NMR of limonene there are two methyl peaks, few CH2 peaks and vinylic protons. In 13-C there are two methyl along with C=C and CH2 peaks.
Want to see more full solutions like this?
Chapter 14 Solutions
A Small Scale Approach to Organic Laboratory Techniques
- ) Specifically, what is the difference between the cyclopentadienyl anion and pyrrole?arrow_forwardExplain why the hydrogen and the methyl substituent are trans to one another after photochemical ring closure of provitamin D3 to form 7-dehydrocholesterol.arrow_forwardCan Gattermann-Koch reaction be considered similar to Friedel Craft’s acylation? Discuss.arrow_forward
- What changes in NMR and IR spectra would you expect to see upon acylation of benzene? Explicitly discuss the elements of the 1H NMR and IR of acetophenone that you would use to argue that the reaction was successful.arrow_forwardExplain the role of trisodium citrate, Na3C6H5O7 as a component of the Benedict’s reagent for the Estimation of Glucosearrow_forwardPlease don't provide handwritten solution. Draw an arrow-pushing mechanism for the Diels Alder reaction of anthracene-9- methanol and N-maleimide.arrow_forward
- Explain why the ERC and ERO of 1,3,5-cycloheptatriene have an equilibrium constant of almost 1. Put another way, why are the ring opening/closing reactions in equilibria at room temperature?arrow_forwardIn the electrophilic aromatic substitution of toluene formation of 4-chlorotoluene is favored over 3-chlorotoluene. Explain this observation by writing the reaction mechanism.?arrow_forwardDescribe how to separate benzoic acid from p-dichlorobenzene using acid base extraction with water and diethyl ether as your solventsarrow_forward
- Briefly explain the electronic effect of the substituent present in p-methoxybenzoic acid as compared to benzoic acid.arrow_forwardInfrared Interpretation – interpret all absorptions in the 4000-1400 cm-1 region of the IR spectra of 2-methyl-4-heptanone. Label the recorded IR spectra and provide an indication of the impurities present, if any.arrow_forwardWhy 9(t-butyl) anthracence has a lower quantum yield than 9-methyl anthracence?arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


