
Concept explainers
Fifty cm3 of 1.000 M nitrous acid is titrated with 0.850 M NaOH. What is the pH of the solution
(a) before any NaOH is added?
(b) at half-neutralization?
(c) at the equivalence point?
(d) when 0.10 mL less than the volume of NaOH to reach the equivalence point is added?
(e) when 0.10 mL more than the volume of NaOH to reach the equivalence point is added?
(f) Use your data to construct a plot similar to that shown in Figure 14.10 (pH versus volume NaOH added).
(a)
Interpretation:
The titration of NaOH of molarity 0.850 M occurs with nitrous acid of 50 cm3 with molarity 1.000M. The pH value of the solution is to be determined before the addition of NaOH.
Concept introduction:
For a reaction as follows:
The equilibrium constant can be calculated as follows:
The pH of the solution can be calculated as follows:
Answer to Problem 74QAP
The pH value of the solution is 1.62.
Explanation of Solution
The dissociation reaction for the nitrous acid is shown below-
Given that-
Molarity of HNO2 = 1.000M
The dissociation constant, Ka = 6.0×10-4
Consider the x M of HNO2 is ionized. Hence the ICE table is shown below-
| HNO2 | H+ | NO2 - | |
| I (mol) | 1.000 | 0 | 0 |
| C (mol) | -x | +x | +x |
| E (mol) | 1.000 -x | x | x |
Now the dissociation constant Ka is calculated as-
Given that-
[HNO2 ] = 1.000 -x
[H+] = x
[NO2 -] = x
Put the above values in equation (1),
On calculation −
x = 2.42×10-2
The concentration of [H+] = x= [NO2 -] = 2.42×10-2 at equilibrium
Now, the pH value is −
The value of pH = 1.62
(b)
Interpretation:
The pH value of the solution is to be determined at half neutralization.
Concept introduction:
The
The pH of the solution can be calculated as follows:
Answer to Problem 74QAP
The pH value of the solution is 3.22 at half neutralization.
Explanation of Solution
At half neutralization, only half acid will be ionized and the equilibrium concentration of the conjugate base of acid and acid will be equal. Therefore,
Or
And
Ka = 6.0×10-4
The value of pH for the solution = 3.22
(c)
Interpretation:
The pH value of the solution is to be determined at the equivalence point.
Concept introduction:
The molarity of solution is calculated as follows:
Here, number of moles is of solute and volume is of solution.
For a base dissociation reaction,
The base dissociation constant will be:
It is related to acid dissocation constant and ionization constant of water as follows:
The pOH and pH of the solution can be calculated as follows:
Here,
Answer to Problem 74QAP
The pH value of the solution is 8.45 at the equivalence point.
Explanation of Solution
The chemical equation for the reaction between nitrous acid and sodium hydroxide is given as-
The net-ionic equation of the above reaction is −
Number of moles of nitrous acid-
Or
Given that-
Molarity = 1.000M
Volume = 50.0cc = 0.050L
Put the above values in equation (1)
Number of moles of HNO2 = 0.0500 mol
At equivalence point-
Number of moles of NaOH added = initial number of moles of HNO2 = 0.0500 mol
Molarity of the base = 0.850 M
The volume of the base-
Put the values in the above equation-
Volume of base = 0.0588 L
Molarity of NO2 - is calculated as-
Number of moles of HNO2 = 0.0500mol = Number of moles of NO2 -
Total volume = 0.0500L +0.0588L
Put the given values in above equation-
Molarity of NO2 - = 0.459M
Consider the M of NO2 - will be ionized. Hence the ICE table is shown below-
| NO2 - | HNO2 | OH- | |
| I (mol) | 0.459 | 0 | 0 |
| C (mol) | -y | +y | +y |
| E (mol) | 0.459 -y | y | y |
Now the base dissociation constant Kb is calculated as-
Given that-
[NO2 -] = 0.459 −y
[HNO2 ] = y
[OH-] = y
Put the above values in equation (2)
On calculation −
[OH-] = y = 2.79×10-6
Now, the pH of the solution −
And,
(d)
Interpretation:
The volume less than the 0.10 mL of NaOH is added to reach the equivalence point. At this condition, the pH value of the solution is to be determined.
Concept introduction:
The molarity of solution is calculated as follows:
Here, number of moles is of solute and volume is of solution.
Now, from the Henderson −Hasselbalch equation is as follows−
Answer to Problem 74QAP
The pH value of the solution is 5.92, when the volume less than the 0.10mL of NaOH added.
Explanation of Solution
The number of moles of base-
Given that-
Volume = 58.7mL (0.1mL less than 58.8mL)
Molarity = 0.850 M
Put the above values in Equ (1)
Number of moles of base (NaOH) = 0.0499mol
Now, the number of moles of HNO2 −
Now, the number of moles of conjugate base, NO2 -
Now, from the Henderson −Hasselbalch equation −
Or,
On calculation-
(e)
Interpretation:
The volume more than the 0.10mL of NaOH is added to reach the equivalence point. At this condition, the pH value of the solution is to be determined.
Concept introduction:
The molarity of solution is calculated as follows:
Here, number of moles is of solute and volume is of solution.
The pH of the solution can be calculated as follows:
Answer to Problem 74QAP
The pH value of the solution is 10.88 when the volume more than the 0.10mL of NaOH added.
Explanation of Solution
The number of moles of base-
Given that-
Volume = 58.9mL (0.1mL greater than 58.8mL)
Molarity = 0.850 M
Put the above values in equation (1)
Number of moles of base (NaOH) = 0.050082mol
Now, the number of moles of OH- −
Now, pOH-
And
(f)
Interpretation:
The graph is to be plotted by using the calculated data between pH vs. NaOH added.
Concept introduction:
The equivalence point is defined as the point for the titration process at which addition of titrant is enough for the complete neutralization of the analyte.
Half neutralization is defined as the point at which the concentration of weak acid will become equal to its conjugate base.
Explanation of Solution
The graph plot between pH vs. NaOH added is shown below-
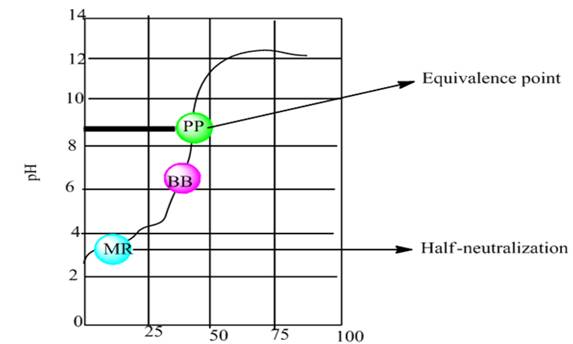
According to the graph, the half neutralization point is approximately at pH 2.8 and equivalence point is approximately at pH 8.8
Want to see more full solutions like this?
Chapter 14 Solutions
Student Solutions Manual For Masterton/hurley's Chemistry: Principles And Reactions, 8th
- Sketch a titration curve for the titration of potassium hydroxide with HCl, both 0.100 M. Identify three regions in which a particular chemical species or system dominates the acid-base equilibria.arrow_forwardA sodium hydrogen carbonate-sodium carbonate buffer is to be prepared with a pH of 9.40. (a) What must the [ HCO3 ]/[ CO32 ]ratio be? (b) How many moles of sodium hydrogen carbonate must be added to a liter of 0.225 M Na2CO3 to give this pH? (c) How many grams of sodium carbonate must be added to 475 mL of 0.336 M NaHCO3 to give this pH? (Assume no volume change.) (d) What volume of 0.200 M NaHCO3 must be added to 735 mL of a 0.139 M solution of Na2CO3 to give this pH? (Assume that volumes are additive.)arrow_forwardWater is accidentally added to 350.00 mL of a stock solution of 6.00 M HCI. A 75.00-mL sample of the diluted solution is titrated to pH 7.00 with 78.8 mL of 4.85 M NaOH. How much water was accidentally added? (Assume that volumes are additive.)arrow_forward
- A solution consisting of 25.00 g NH4Cl in 178 mL of water is titrated with 0.114 M KOH. (a) How many milliliters of KOH are required to reach the equivalence point? (b) Calculate [Cl-], [K+], [NH3], and [OH-] at the equivalence point. (Assume that volumes are additive.) (c) What is the pH at the equivalence point?arrow_forwardA chemist needs a buffer with pH 3.50. How many milliliters of pure formic acid (density = 1.220 g/mL) must be added to 375 mL of 0.0857 M NaOH solution to obtain such a buffer?arrow_forwardA 25.0-mL sample of hydroxylamine is titrated to the equivalence point with 35.8 mL of 0.150 M HCl. a What was the concentration of the original hydroxylamine solution? b What is the pH at the equivalence point? c Which indicators, bromphenol blue, methyl red, or phenolphthalein, should be used to detect the end point of the titration? Why?arrow_forward
- The species called glacial acetic acid is 98% acetic acid by mass (d=1.0542g/mL). What volume of glacial acetic acid must be added to 100.0 mL of 1.25 M NaOH to give a buffer with a pH of 4.20?arrow_forwardA buffer is prepared by dissolving 0.0250 mol of sodium nitrite, NaNO2, in 250.0 mL of 0.0410 M nitrous acid, HNO2. Assume no volume change after HNO2 is dissolved. Calculate the pH of this buffer.arrow_forwardWhat is the pH of the solution obtained by titrating 1.30 g of sodium hydrogen sulfate, NaHSO4, dissolved in 50.0 mL of water with 0.175 M sodium hydroxide until the equivalence point is reached? Assume that any volume change due to adding the sodium hydrogen sulfate or to mixing the solutions is negligible.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





