
Fundamentals of Chemical Engineering Thermodynamics (MindTap Course List)
1st Edition
ISBN: 9781111580704
Author: Kevin D. Dahm, Donald P. Visco
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14.7, Problem 18P
Reactants A and B combine to form product P in the liquid phase reaction:
A + B ↔ P
But P reacts further to form an undesired by-product U:
2P ↔ U
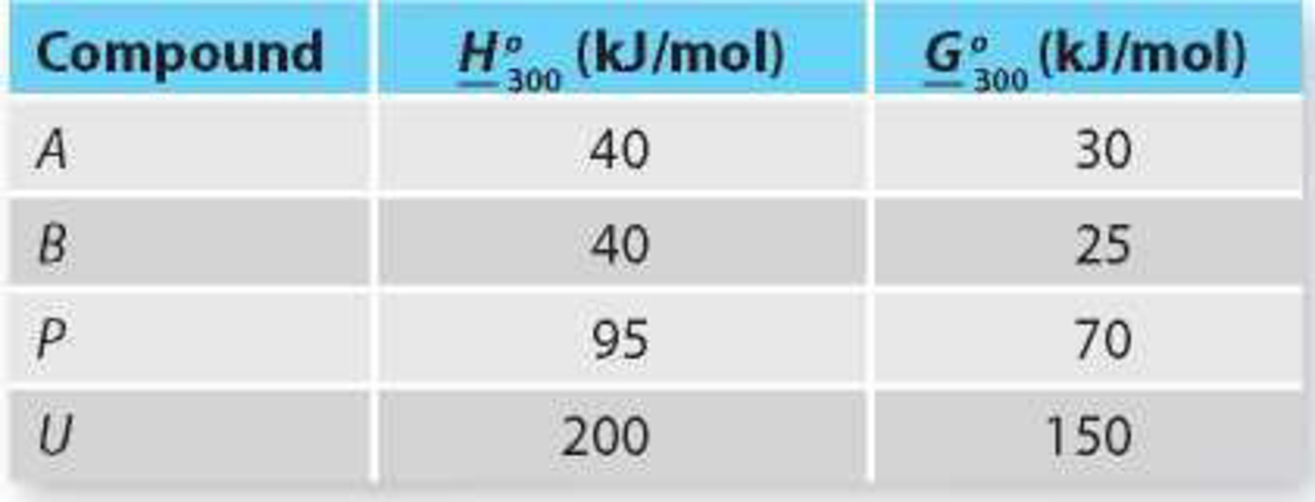
The Gibbs free energy and enthalpy of each compound at T = 300 K and P = 1 bar are given in Table P14-18. It is reasonable to assume that both reactions have ΔCP = 0 and that A, B, P, and U form ideal solutions.
The feed entering a steady-state reactor is 1000 mol/hr each of compounds A and B. The reactor is at a uniform pressure of 1 bar.
- A. Determine the equilibrium composition of the exit stream at 300 K.
- B. Determine the equilibrium composition of the exit stream at 600 K.
- C. The second reaction has a larger equilibrium constant than the first, yet there is, at equilibrium, more P than U. Why?
TABLE P14-18
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Fundamentals of Chemical Engineering Thermodynamics (MindTap Course List)
Ch. 14.6 - Determine the equilibrium constant at T = 298.15 K...Ch. 14.6 - Determine the equilibrium constant at T = 298.15 K...Ch. 14.6 - Determine the equilibrium constant at T = 298.15 K...Ch. 14.6 - Prob. 4ECh. 14.6 - Prob. 5ECh. 14.6 - Prob. 6ECh. 14.6 - Prob. 8ECh. 14.6 - For the following gas phase reaction,...Ch. 14.6 - Prob. 12ECh. 14.7 - A closed system reactor initially contains two...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=J4WJCYpTYj8;License: Standard Youtube License