
INTRO.TO CHEM.ENGR.THERMO.-EBOOK>I<
8th Edition
ISBN: 9781260940961
Author: SMITH
Publisher: INTER MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15, Problem 15.20P
Estimate the solubility of naphthalene! 1) in nitrogen(2) at a temperature of 35°C at pressures up to 300 bar. Use the procedure described in Sec. 15.4, with
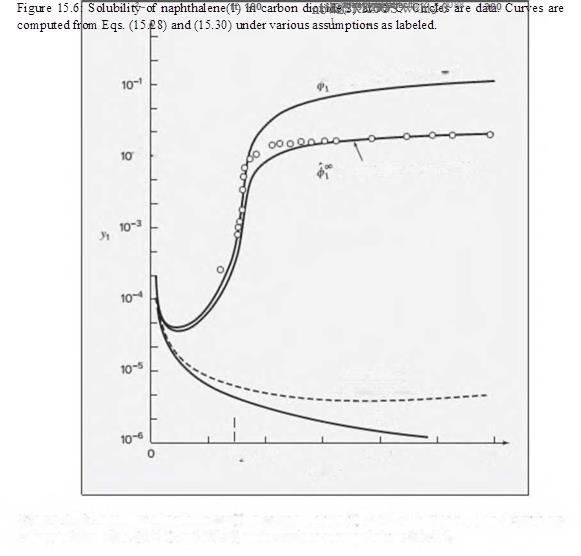
Figure 15.6: Solubility of naphthalene(1) in carbon dioxide(2) at 35°C. Circles are data. Curves are computed from Eqs. (15.28) and (15.30) under various assumptions as labeled.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
INTRO.TO CHEM.ENGR.THERMO.-EBOOK>I<
Ch. 15 - Prob. 15.1PCh. 15 - Prob. 15.2PCh. 15 - Work Prob. 15.2 for the van Laar equation. 15.2. A...Ch. 15 - Consider a binary vapor-phase mixture described by...Ch. 15 - Prob. 15.5PCh. 15 - It has been suggested that a value for GEof at...Ch. 15 - Pure liquid species 2 and 3 are for practical...Ch. 15 - It is demonstrated in Ex. 15.5 that the Wilson...Ch. 15 - Vapor sulfur hexafluoride SF6 at pressures of...Ch. 15 - Prob. 15.10P
Ch. 15 - Toluene(l) and water (2) are essentially...Ch. 15 - n-Heptane(l) and water (2) are essentially...Ch. 15 - Consider a binary system of species 1 and 2 in...Ch. 15 - Prob. 15.14PCh. 15 - 15.15. Work the preceding problem for mole...Ch. 15 - The Case I behavior for SLE (Sec. 15.4) has an...Ch. 15 - Prob. 15.17PCh. 15 - Prob. 15.18PCh. 15 - Prob. 15.19PCh. 15 - Estimate the solubility of naphthalene! 1) in...Ch. 15 - Prob. 15.21PCh. 15 - 15.22. The UNILAN equation for pure-species...Ch. 15 - In Ex. 15.8, Henry's constant for adsorption k,...Ch. 15 - It was assumed in the development of Eq. (15.39)...Ch. 15 - Suppose that the adsorbate equation of state is...Ch. 15 - Suppose that the adsorbate equation of state is...Ch. 15 - Derive the result given in the third step of the...Ch. 15 - Consider a ternary system comprising solute...Ch. 15 - It is possible in principle for a binary liquid...Ch. 15 - With V2=V2 , Eq.(15.66) for the osmotic pressure...Ch. 15 - A liquid-process feed stream F contains 99 mol-%...Ch. 15 - At 25°C the solubility of n-hexane in water is 2...Ch. 15 - A binary liquid mixture is only partially miscible...Ch. 15 - The spinodal curve for a binary liquid system is...Ch. 15 - Prob. 15.36PCh. 15 - Many fluids could be used as solvent species for...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The
Homogeneous and Heterogeneous Equilibrium - Chemical Equilibrium - Chemistry Class 11; Author: Ekeeda;https://www.youtube.com/watch?v=8V9ozZSKl9E;License: Standard YouTube License, CC-BY