
(a)
Interpretation:
To graph the figures for
Concept Introduction:
Plot A v/s T by varying T and finding respective values of A
Plot T v/s x,by varying T, find respective values of x using the below equation,
Where,
T = TemperaturE
x = Mole fraction
UCST = Upper Critical Solution TemperaturE
LCST = Lower Critical Solution TemperaturE
(a)
Answer to Problem 15.5P
The corresponding figures of A v/s T and T v/s x are plotted.
Explanation of Solution
Using the above equation and varying T,
Now, Plot A v/s T, we get,
| T | A | T | A | T | A | T | A |
| 250 | 2.064383 | 305 | 1.957657 | 355 | 1.962832 | 405 | 2.019069 |
| 255 | 2.04732 | 310 | 1.954878 | 360 | 1.966645 | 410 | 2.02652 |
| 260 | 2.032045 | 315 | 1.952956 | 365 | 1.970925 | 415 | 2.034233 |
| 265 | 2.018435 | 320 | 1.951838 | 370 | 1.975644 | 420 | 2.042193 |
| 270 | 2.006377 | 325 | 1.951476 | 375 | 1.980778 | 425 | 2.050385 |
| 275 | 1.995768 | 330 | 1.951823 | 380 | 1.986303 | 430 | 2.058797 |
| 280 | 1.986512 | 335 | 1.952839 | 385 | 1.992198 | 435 | 2.067417 |
| 285 | 1.97852 | 340 | 1.954484 | 390 | 1.99844 | 440 | 2.076233 |
| 290 | 1.971712 | 345 | 1.95672 | 395 | 2.005012 | 445 | 2.085234 |
| 295 | 1.966011 | 350 | 1.959514 | 400 | 2.011894 | 450 | 2.094409 |
| 300 | 1.961347 | 455 | 2.103749 |
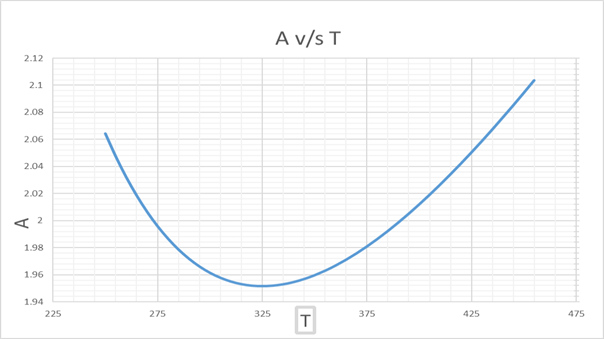
From this graph of A v/s T, we get,
A =2 at T = 272.92 K and 391.209 K
Hence, we get that, UCST = 272.92 K
and
LCST = 391.2 K
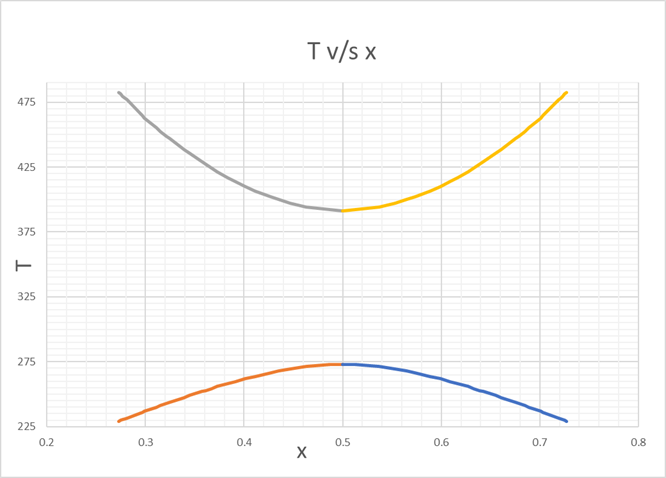
Since, There are 2 points for A =2, We can plot T v/s x
Varying T from
225 K to UCST
And from
LCST to 482 K
For finding x,
Varying, T, we find the values of x respectively upto 3 decimals.
We can see that the values of T are same for x and (1-x).
We find the corresponding values, for T v/s x, as below,
| x | A | x | A | A | T |
| 0.273 | 2.157389 | 0.727 | 2.157389 | 2.157711656 | 229.2 |
| 0.275 | 2.154223 | 0.725 | 2.154223 | 2.15444794 | 229.8 |
| 0.277 | 2.151103 | 0.723 | 2.151103 | 2.15122162 | 230.4 |
| 0.279 | 2.148026 | 0.721 | 2.148026 | 2.148032352 | 231 |
| 0.281 | 2.144992 | 0.719 | 2.144992 | 2.144879797 | 231.6 |
| 0.295 | 2.124933 | 0.705 | 2.124933 | 2.125260129 | 235.5 |
| 0.297 | 2.122228 | 0.703 | 2.122228 | 2.122372431 | 236.1 |
| 0.299 | 2.119563 | 0.701 | 2.119563 | 2.119518699 | 236.7 |
| 0.311 | 2.104361 | 0.689 | 2.104361 | 2.10441677 | 240 |
| 0.315 | 2.099584 | 0.685 | 2.099584 | 2.099167952 | 241.2 |
| 0.321 | 2.092682 | 0.679 | 2.092682 | 2.092783653 | 242.7 |
| 0.325 | 2.08825 | 0.675 | 2.08825 | 2.089045662 | 243.6 |
| 0.339 | 2.073769 | 0.661 | 2.073769 | 2.073625685 | 247.5 |
| 0.345 | 2.068035 | 0.655 | 2.068035 | 2.06802134 | 249 |
| 0.357 | 2.057374 | 0.643 | 2.057374 | 2.057334882 | 252 |
| 0.361 | 2.054052 | 0.639 | 2.054052 | 2.054261227 | 252.9 |
| 0.367 | 2.04928 | 0.633 | 2.04928 | 2.049270664 | 254.4 |
| 0.373 | 2.044756 | 0.627 | 2.044756 | 2.044442293 | 255.9 |
| 0.383 | 2.037752 | 0.617 | 2.037752 | 2.037948522 | 258 |
| 0.391 | 2.032618 | 0.609 | 2.032618 | 2.032623149 | 259.8 |
| 0.401 | 2.026769 | 0.599 | 2.026769 | 2.026683214 | 261.9 |
| 0.411 | 2.021534 | 0.589 | 2.021534 | 2.02181967 | 263.7 |
| 0.427 | 2.014395 | 0.573 | 2.014395 | 2.014166319 | 266.7 |
| 0.435 | 2.011382 | 0.565 | 2.011382 | 2.011259023 | 267.9 |
| 0.447 | 2.007542 | 0.553 | 2.007542 | 2.007745467 | 269.4 |
| 0.463 | 2.003663 | 0.537 | 2.003663 | 2.003702412 | 271.2 |
| 0.487 | 2.000451 | 0.513 | 2.000451 | 2.000474405 | 272.7 |
| x | A | x | A | A | T |
| 0.273 | 2.157389 | 0.727 | 2.157389 | 2.1576682 | 482.5 |
| 0.275 | 2.154223 | 0.725 | 2.154223 | 2.1556408 | 481.5 |
| 0.277 | 2.151103 | 0.723 | 2.151103 | 2.1515998 | 479.5 |
| 0.279 | 2.148026 | 0.721 | 2.148026 | 2.1483803 | 477.9 |
| 0.281 | 2.144992 | 0.719 | 2.144992 | 2.1465746 | 477 |
| 0.295 | 2.124933 | 0.705 | 2.124933 | 2.1248316 | 466 |
| 0.297 | 2.122228 | 0.703 | 2.122228 | 2.1221099 | 464.6 |
| 0.299 | 2.119563 | 0.701 | 2.119563 | 2.1190127 | 463 |
| 0.311 | 2.104361 | 0.689 | 2.104361 | 2.1046921 | 455.5 |
| 0.315 | 2.099584 | 0.685 | 2.099584 | 2.0990594 | 452.5 |
| 0.321 | 2.092682 | 0.679 | 2.092682 | 2.0925609 | 449 |
| 0.325 | 2.08825 | 0.675 | 2.08825 | 2.0888838 | 447 |
| 0.339 | 2.073769 | 0.661 | 2.073769 | 2.0735686 | 438.5 |
| 0.345 | 2.068035 | 0.655 | 2.068035 | 2.0682904 | 435.5 |
| 0.357 | 2.057374 | 0.643 | 2.057374 | 2.057098 | 429 |
| 0.361 | 2.054052 | 0.639 | 2.054052 | 2.0545646 | 427.5 |
| 0.367 | 2.04928 | 0.633 | 2.04928 | 2.0495558 | 424.5 |
| 0.373 | 2.044756 | 0.627 | 2.044756 | 2.0446266 | 421.5 |
| 0.383 | 2.037752 | 0.617 | 2.037752 | 2.0373882 | 417 |
| 0.391 | 2.032618 | 0.609 | 2.032618 | 2.0326704 | 414 |
| 0.401 | 2.026769 | 0.599 | 2.026769 | 2.0265203 | 410 |
| 0.411 | 2.021534 | 0.589 | 2.021534 | 2.0212758 | 406.5 |
| 0.427 | 2.014395 | 0.573 | 2.014395 | 2.0147294 | 402 |
| 0.435 | 2.011382 | 0.565 | 2.011382 | 2.0118936 | 400 |
| 0.447 | 2.007542 | 0.553 | 2.007542 | 2.0077282 | 397 |
| 0.463 | 2.003663 | 0.537 | 2.003663 | 2.003672 | 394 |
| 0.5 | 2 | 0.5 | 2 | 2 | 391.2092 |
(b)
Interpretation:
To graph the figures for
Concept Introduction:
Plot A v/s T by varying T and finding respective values of A
Plot T v/s x, by varying T, find respective values of x using the below equation,
Where,
T = TemperaturE
x = Mole fraction
UCST = Upper Critical Solution TemperaturE
LCST = Lower Critical Solution TemperaturE
(b)
Answer to Problem 15.5P
The corresponding figures of A v/s T and T v/s x are plotted.
Explanation of Solution
Using the above equation and varying T,
Now, Plot A v/s T, we get,
| T | A | T | A | T | A | T | A |
| 250 | 1.624382754 | 305 | 1.831427133 | 355 | 2.037480129 | 405 | 2.244994535 |
| 255 | 1.641437694 | 310 | 1.851652376 | 360 | 2.058312094 | 410 | 2.26554465 |
| 260 | 1.65896797 | 315 | 1.872003631 | 365 | 2.079144116 | 415 | 2.28604038 |
| 265 | 1.676925327 | 320 | 1.892462987 | 370 | 2.099968476 | 420 | 2.30647842 |
| 270 | 1.695265877 | 325 | 1.913014009 | 375 | 2.120778078 | 425 | 2.326855742 |
| 275 | 1.713949657 | 330 | 1.9336416 | 380 | 2.14156639 | 430 | 2.34716958 |
| 280 | 1.732940238 | 335 | 1.954331894 | 385 | 2.162327405 | 435 | 2.367417404 |
| 285 | 1.752204383 | 340 | 1.975072147 | 390 | 2.183055602 | 440 | 2.387596908 |
| 290 | 1.771711734 | 345 | 1.995850642 | 395 | 2.203745902 | 445 | 2.407705993 |
| 295 | 1.791434544 | 350 | 2.016656606 | 400 | 2.224393641 | 450 | 2.427742748 |
| 300 | 1.811347424 | 455 | 2.447705444 |
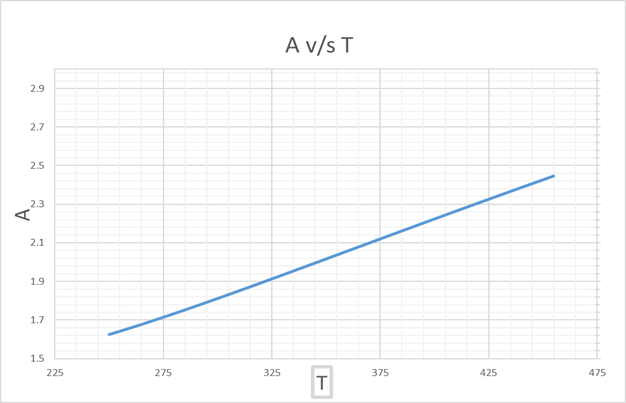
From this graph of A v/s T, we get,
A =2 at T = 346 K
Hence, we get that,
LCST = 391.2 K
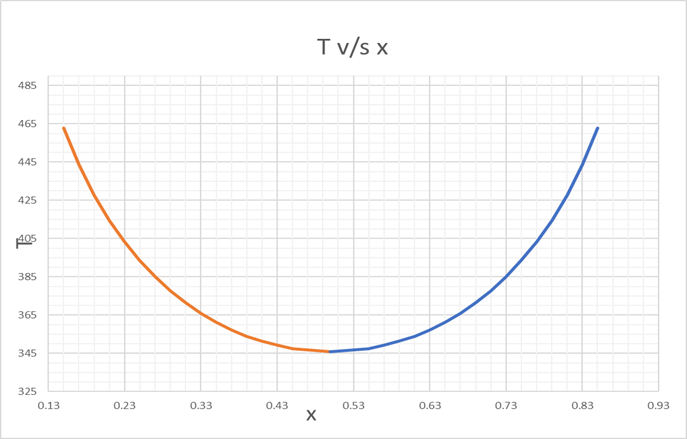
Since, there is only 1 point for A =2, we can plot T v/s x
And from
LCST to 482 K
For finding x,
Varying, T, we find the values of x respectively upto 3 decimals.
We can see that the values of T are same for x and (1-x).
We find the corresponding values, for T v/s x, as below,
| x | A | x | A | A | T |
| 0.15 | 2.478002 | 0.85 | 2.478002 | 2.4783 | 462.7 |
| 0.17 | 2.402466 | 0.83 | 2.402466 | 2.402485 | 443.7 |
| 0.19 | 2.338726 | 0.81 | 2.338726 | 2.338239 | 427.8 |
| 0.21 | 2.284354 | 0.79 | 2.284354 | 2.284403 | 414.6 |
| 0.23 | 2.237613 | 0.77 | 2.237613 | 2.237938 | 403.286 |
| 0.25 | 2.197225 | 0.75 | 2.197225 | 2.197543 | 393.5 |
| 0.27 | 2.162223 | 0.73 | 2.162223 | 2.162327 | 385 |
| 0.29 | 2.131867 | 0.71 | 2.131867 | 2.131591 | 377.6 |
| 0.31 | 2.105577 | 0.69 | 2.105577 | 2.105381 | 371.3 |
| 0.33 | 2.082897 | 0.67 | 2.082897 | 2.082893 | 365.9 |
| 0.35 | 2.063464 | 0.65 | 2.063464 | 2.063729 | 361.3 |
| 0.37 | 2.046988 | 0.63 | 2.046988 | 2.046771 | 357.23 |
| 0.39 | 2.033237 | 0.61 | 2.033237 | 2.033314 | 354 |
| 0.41 | 2.02203 | 0.59 | 2.02203 | 2.022069 | 351.3 |
| 0.43 | 2.013223 | 0.57 | 2.013223 | 2.013326 | 349.2 |
| 0.45 | 2.006707 | 0.55 | 2.006707 | 2.006251 | 347.5 |
| 0.47 | 2.002405 | 0.53 | 2.002405 | 2.002922 | 346.7 |
| 0.49 | 2.000267 | 0.51 | 2.000267 | 2.000634 | 346.15 |
| 0.5 | 2 | 0.5 | 2 | 2.00001 | 346 |
(c)
Interpretation:
To graph the figures for
Concept Introduction:
Plot A v/s T by varying T and finding respective values of A
Plot T v/s x, by varying T, find respective values of x using the below equation,
Where,
T = TemperaturE
x = Mole fraction
UCST = Upper Critical Solution TemperaturE
LCST = Lower Critical Solution TemperaturE
(c)
Answer to Problem 15.5P
The corresponding figures of A v/s T and T v/s x are plotted.
Explanation of Solution
Using the above equation and varying T,
Now, Plot A v/s T, we get,
| T | A | T | A | T | A | T | A |
| 250 | 2.664382754 | 305 | 2.178968117 | 355 | 1.941705481 | 405 | 1.815364905 |
| 255 | 2.606143577 | 310 | 2.14842657 | 360 | 1.924978761 | 410 | 1.807008064 |
| 260 | 2.551275662 | 315 | 2.119622678 | 365 | 1.909281102 | 415 | 1.799293392 |
| 265 | 2.499566836 | 320 | 2.092462987 | 370 | 1.894563071 | 420 | 1.792192705 |
| 270 | 2.450821433 | 325 | 2.066860162 | 375 | 1.880778078 | 425 | 1.785679271 |
| 275 | 2.404858748 | 330 | 2.042732509 | 380 | 1.867882179 | 430 | 1.779727719 |
| 280 | 2.361511667 | 335 | 2.020003536 | 385 | 1.855833899 | 435 | 1.774313955 |
| 285 | 2.320625436 | 340 | 1.998601559 | 390 | 1.844594064 | 440 | 1.76941509 |
| 290 | 2.282056562 | 345 | 1.978459338 | 395 | 1.834125649 | 445 | 1.765009363 |
| 295 | 2.245671832 | 350 | 1.959513749 | 400 | 1.824393641 | 450 | 1.761076082 |
| 300 | 2.211347424 | 455 | 1.757595554 |
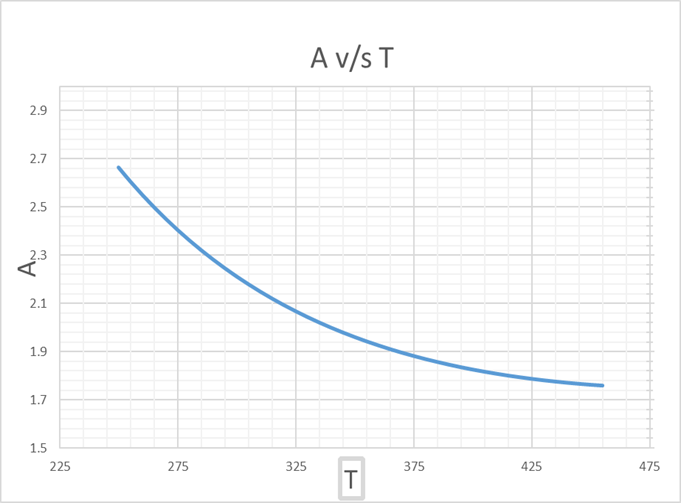
From this graph of A v/s T, we get,
A =2 at T = 339.66 K
Hence, we get that,
UCST = 339.66 K
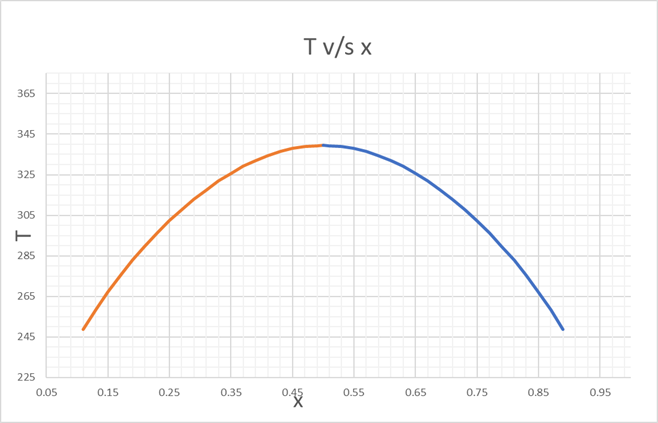
Since, there is only 1 point for A =2, we can plot T v/s x
And from
250 K to UCST
For finding x,
Varying, T, we find the values of x respectively up to 3 decimals.
We can see that the values of T are same for x and (1-x).
We find the corresponding values, for T v/s x, as below,
| x | A | x | A | A | T |
| 0.11 | 2.680437 | 0.89 | 2.680437 | 2.68011 | 248.7 |
| 0.13 | 2.568863 | 0.87 | 2.568863 | 2.56848 | 258.4 |
| 0.15 | 2.478002 | 0.85 | 2.478002 | 2.47874 | 267.1 |
| 0.17 | 2.402466 | 0.83 | 2.402466 | 2.40219 | 275.3 |
| 0.19 | 2.338726 | 0.81 | 2.338726 | 2.33801 | 282.838 |
| 0.21 | 2.284354 | 0.79 | 2.284354 | 2.28431 | 289.7 |
| 0.23 | 2.237613 | 0.77 | 2.237613 | 2.23725 | 296.2 |
| 0.25 | 2.197225 | 0.75 | 2.197225 | 2.19687 | 302.2 |
| 0.27 | 2.162223 | 0.73 | 2.162223 | 2.16225 | 307.7 |
| 0.29 | 2.131867 | 0.71 | 2.131867 | 2.13151 | 312.9 |
| 0.31 | 2.105577 | 0.69 | 2.105577 | 2.10552 | 317.56 |
| 0.33 | 2.082897 | 0.67 | 2.082897 | 2.08204 | 322 |
| 0.35 | 2.063464 | 0.65 | 2.063464 | 2.0634 | 325.7 |
| 0.37 | 2.046988 | 0.63 | 2.046988 | 2.0464 | 329.22 |
| 0.39 | 2.033237 | 0.61 | 2.033237 | 2.03348 | 332 |
| 0.41 | 2.02203 | 0.59 | 2.02203 | 2.02266 | 334.4 |
| 0.43 | 2.013223 | 0.57 | 2.013223 | 2.01345 | 336.5 |
| 0.45 | 2.006707 | 0.55 | 2.006707 | 2.00658 | 338.1 |
| 0.47 | 2.002405 | 0.53 | 2.002405 | 2.00278 | 339 |
| 0.49 | 2.000267 | 0.51 | 2.000267 | 2.00194 | 339.2 |
| 0.5 | 2 | 0.5 | 2 | 339.663 |
Want to see more full solutions like this?
Chapter 15 Solutions
GEN, ORG & BIOL CHEM: CUSTOM SSC
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





