
Concept explainers
(a)
Interpretation:
Molar concentration of each ion found after
Concept Introduction:
Molarity is quantitatively defined as moles of solute in one liter of solution. For example
Where,
(a)
Explanation of Solution
Since in
Similarly for other solution, in
Total moles of solution when two
Total volume of solution when two
The formula to evaluate volume from molarity is given as follows:
Substitute
Since
Since
Hence molar concentration of both
(b)
Interpretation:
Molar concentration of each ion found after
Concept Introduction:
Refer to part (a).
(b)
Explanation of Solution
Since in
Similarly for other solution, in
Total volume of solution upon when two
Substitute
Substitute
Thus total molar concentration of
Since
Since
Hence molar concentration of both
(c)
Interpretation:
Molar concentration each ion found after
Concept Introduction:
Refer to part (a).
(c)
Explanation of Solution
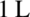 the moles of
the moles of
Since in
Since in
Since
Since
Since
Since
Thus total molar concentration of
Hence molar concentration of
(d)
Interpretation:
Molar concentration of each ion found after
Concept Introduction:
Refer to part (a).
(d)
Explanation of Solution
Since in
Since in
Since
Since
Since
Since
Hence molar concentration of
Want to see more full solutions like this?
Chapter 15 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
- Lead poisoning has been a hazard for centuries. Some scholars believe that the decline of the Roman Empire can be traced, in part, to high levels of lead in water from containers and pipes, and from wine that was stored in leadglazed containers. If we presume that the typical Roman water supply was saturated with lead carbonate, PbCO3 (Ksp = 7.4 1014), how much lead will a Roman ingest in a year if he or she drinks 1 L/day from the container?arrow_forwardWrite a net ionic equation for any precipitation reaction that occurs when 1 M solutions of the following are mixed. (a) copper(II) sulfate and sodium chloride (b) manganese(II) nitrate and ammonium hydroxide (c) silver nitrate and hydrochloric acid (d) nickel(II) sulfate and potassium hydroxide (e) ammonium carbonate and sodium nitratearrow_forwardCalcium carbonate, CaCO3, can be obtained in a very pure state. Standard solutions of calcium ion are usually prepared by dissolving calcium carbonate in acid. What mass of CaCO3 should be taken to prepare 500. mL of 0.0200 M calcium ion solution?arrow_forward
- Describe in words how you would prepare pure crystalline AgCl and NaNO3 from solid AgNO3 and solid NaCl.arrow_forwardEqual quantities of the hypothetical strong acid HX, weak acid HA, and weak base BZ are added to separate beakers of water, producing the solutions depicted in the drawings. In the drawings, the relative amounts of each substance present in the solution (neglecting the water) are shown. Identify the acid or base that was used to produce each of the solutions (HX, HA, or BZ).arrow_forwardOranges and grapefruits are known as citrus fruits because their acidity comes mainly from citric acid, H3C6H5O7. Calculate the concentration of citric acid in a solution if a 30.00-mL sample is neutralized by 15.10 mL of 0.0100 M KOH. Assume that three acidic hydrogens of each citric acid molecule are neutralized in the reaction.arrow_forward
- A student tries to determine experimentally the molar mass of aspirin (HAsp). She takes 1.00 g of aspirin, dissolves it in water, and neutralizes it with 17.6 mL of 0.315 M KOH. The equation for the reaction is HAsp(aq)+OH(aq)Asp(aq)+H2OWhat is the molar mass of aspirin?arrow_forwardFollow the directions of Question 7 for solutions of the following: (a) silver nitrate and sodium chloride (b) cobalt(II) nitrate and sodium hydroxide (c) ammonium phosphate and potassium hydroxide (d) copper(II) sulfate and sodium carbonate (e) lithium sulfate and barium hydroxidearrow_forwardThe molarity of iodine in solution can be determined by titration with arsenious acid, H3AsO4. The unbalanced equation for the reaction is H3AsO3(aq)+I2(aq)+H2O2 I(aq)+H3AsO4(aq)+2 H+(aq)A 243-mL solution of aqueous iodine is prepared by dissolving iodine crystals in water. A fifty-mL portion of the solution requires 15.42 mL of 0.134 M H3AsO3 for complete reaction. What is the molarity of the solution? How many grams of iodine were added to the solution?arrow_forward
- Write molecular and net ionic equations for the successive neutralizations of each acidic hydrogen of sulfurous acid by aqueous calcium hydroxide. CaSO3 is insoluble; the acid salt is soluble.arrow_forward3.85 The particulate drawing shown represents an aqueous so- lution of an acid HA, where A might represent an atom or group of atoms. Is HA a strong acid or a weak acid? Explain how you can tell from the picture.arrow_forwardTwo liters of a 1.5 M solution of sodium hydroxide are needed for a laboratory experiment. A stock solution of 5.0 M NaOH is available. How is the desired solution prepared?arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





