
(a)
Interpretation:
The weight average molecular weight and degree of polymerization for the given polymer are to be calculated.
Concept Introduction:
The molecular weight of a polymer is not fixed. It has a certain range under which its molecular weight falls. For a linear polymer, degree of polymerization represents the average length of the polymer. Degree of polymerization is defined as:
Weight average molecular weight
Here,
Answer to Problem 16.15P
The weight average molecular weight for the given polymer is
The degree of polymerization for the given polymer is
Explanation of Solution
Given information:
A sample of polyacrylonitrile was analyzed and the results are:
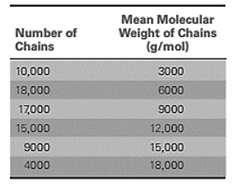
First calculate the total number of chains as:
Now, calculate the weight for each of the chain category as:
Calculate the total weight of all the chains as:
Calculate the mass fraction for each of the size range as:
Now, use formula (2) to calculate the weight average molecular weight of the polymer sample as:
The repeating unit of the polymer polyacrylonitrile is
Now, use equation (1) and calculate the degree of polymerization as:
(b)
Interpretation:
The number average molecular weight and degree of polymerization for the given polymer are to be calculated.
Concept Introduction:
The molecular weight of a polymer is not fixed. It has a certain range under which its molecular weight falls. For a linear polymer, degree of polymerization represents the average length of the polymer. Degree of polymerization is defined as:
Number average molecular weight
Here,
Answer to Problem 16.15P
The number average molecular weight for the given polymer is
The degree of polymerization for the given polymer is
Explanation of Solution
Given information:
A sample of polyacrylonitrile was analyzed and the results are:
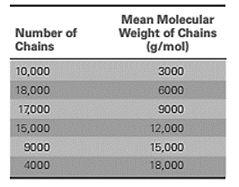
First calculate the total number of chains as:
Calculate the fraction of chain in each of the size range as:
Now, use formula (3) to calculate the number average molecular weight of the polymer sample as:
The repeating unit of the polymer polyacrylonitrile is
Now, use equation (1) and calculate the degree of polymerization as:
Want to see more full solutions like this?
Chapter 16 Solutions
ESS.MAT.SCI (LL W/MINDTAP)
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





