
(a)
Interpretation:
To interpret the value of ideal power required5
Concept Introduction:
The initial stage contains liquid water at
Answer to Problem 16.1P
The ideal power required
Explanation of Solution
At initial stage,
At final stage,
The value of
The flowsheet of the given process is as follows,
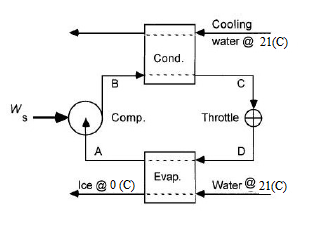
The various positions in the flowsheet are described as follows,
Point A: saturated vapor at
Point B: Superheated vapor at
Point C: saturated liquid at
Point D: Mixture of saturated liquid & saturated vapor at
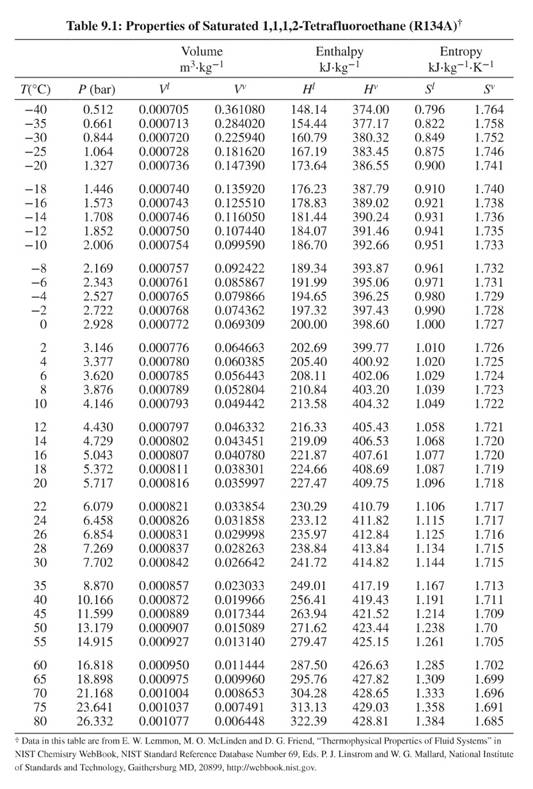
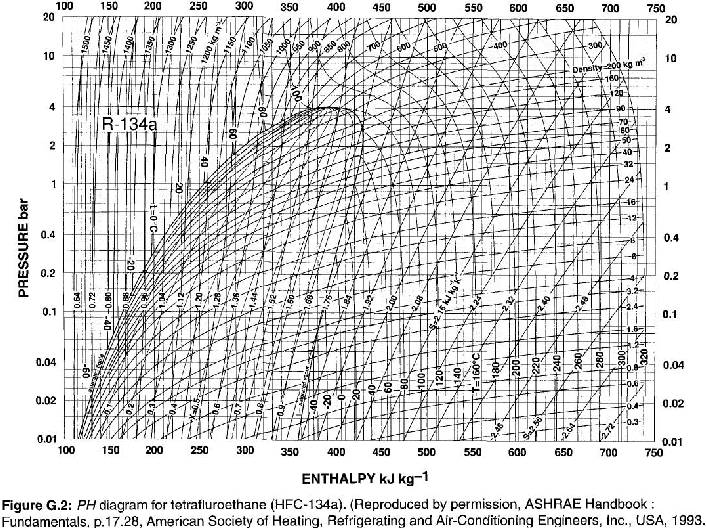
Data for Points A, C, & D from Table 9.1. Data for Point B from Fig. G.2. For reference, Table 9.1 and Figure G.2 are below.
The equation to calculate the ideal work done
Upon substituting the values,
Let the mass flow rate be
So, the ideal power required is
So, the ideal power required is
(b)
Interpretation:
To calculate the ideal power required
Concept Introduction:
A single Carnot heat pump is operated between sink and source at
Answer to Problem 16.1P
The ideal power required
Explanation of Solution
For the Carnot heat pump, heat equal to the enthalpy change of the water is extracted from a cold reservoir at
The given data is as follows,
The heat removed is
The work done can be calculated as
The power required is
The thermodynamic efficiency is
©
Interpretation:
To interpret the power requirement of an ideal tetrafluoroethane vapor-compression refrigeration cycle and thermodynamic efficiency of the process.
Concept Introduction:
Vapor-compression refrigeration cycle is operated using tetrafluoroethane. Ideal condition implies Isentropic Compression, Infinite cooling water rate in the condenser and minimum heat transfer driving forces in evaporator and condenser.
Answer to Problem 16.1P
The power requirement of an ideal tetrafluoroethane vapor-compression refrigeration cycle is
Explanation of Solution
Conventional refrigeration cycle under ideal conditions of operation: Isentropic compression, infinite flow rate of cooling water, & minimum temperature difference for heat transfer = 0.
The data is as follows,
For saturated liquid and vapor at
For saturated liquid at
For superheated vapor at
Refrigeration Circulation Rate:
Substitute the values in the above equations,
(d)
Interpretation:
To interpret the power requirement
Concept Introduction:
Vapor-compression refrigeration cycle is operated using tetrafluoroethane. The thermodynamic efficiency is
Answer to Problem 16.1P
The power requirement
Explanation of Solution
Given, efficiency is
The practical cycle has 4 major points,
Point A: Saturated vapor at
Point B: Superheated vapor at
Point C: Saturated Liquid at
Point D: Mix of saturated liquid and saturated vapor at
For saturated liquid and vapor at
For saturated liquid at
For isentropic compression, the entropy of Point B is
Entropy at point D can be calculated as
\n
Refrigerant circulation rate can be calculated as
\n
Thermodynamic Analysis
\nHere
Want to see more full solutions like this?
Chapter 16 Solutions
Introduction to Chemical Engineering Thermodynamics
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





