
(a)
Interpretation:
The given reaction is
Both compounds has to be showed as terpenes.
Concept Introduction:
Terpene:
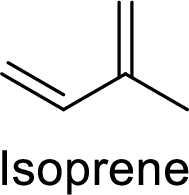
The compounds which contains two or more isoprene units in their structure are said to be terpenes.
Isoprene (2-methyl-1,3-butadiene) unit is a molecule with five carbon atoms with double bonds.
(b)
Interpretation:
A mechanism for the conversion of (S)-Citronellal to Isopulegol has to be proposed.
(c)
Interpretation:
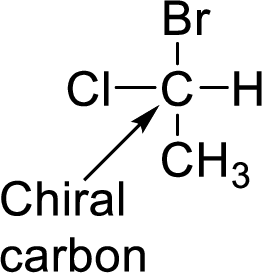
In Isopulegol, the number of stereocenters present has to be given and the number of stereoisomers possible for a molecule with this number of stereocenters has to be given.
Concept Introduction:
A carbon which is bonded to four different groups is said to be chiral carbon. A chiral carbon in a molecule is said to be chiral center. All chiral centers are stereocenters.
(d)
Interpretation:
Isopulegol is formed as a single stereoisomer. The fact that only single stereoisomer is formed has to be accounted.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Student Study Guide and Solutions Manual for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Show how you would prepare cyclopentene from each compound.(a) cyclopentanol(b) cyclopentyl bromidearrow_forwardAn unknown compound A of molecular formula C10H18O reacts with H2SO4 to form two compounds (B and C)of molecular formula C10H16. B and C both react with H2 in the presence of Pd-C to form decalin. Ozonolysis of B forms D, and ozonolysis of C forms a diketone E of molecular formula C10H16O2. Identify the structures of compounds A, B, C, and E.arrow_forward(c) Answer each of the questions below that relate to acetophenone: Xo (i) (ii) (iii) Draw the structure of the enol form of acetophenone. Give a stepwise mechanism for the conversion of acetophenone into its enol form. Show how each of the three compounds A, B and C below can be prepared from acetophenone. Explain clearly what reactants/reagents would be required in each case. odocor A B Br Carrow_forward
- The (R)-isomer of a-terpineol is a component of perfumes and flavorings and has a lilac-like floral odor. The (S)-isomer has a pine-like odor. Propose two methods of producing a-terpineol using Grignard reactions. [Ignore stereochemistry in your synthesises.]arrow_forwardPredict the major products formed when benzoyl chloride (PhCOCl) reacts with the following reagents.(a) ethanol (b) sodium acetate (c) anilinearrow_forwardShow how you would synthesize octanal from each compound. You may use any necessary reagents.(a) octan-1-olarrow_forward
- Compound A has molecular formula C7H15B.. Treatment of compound A with sodium ethoxide yields only one elimination product (compound B) and no substitution products. When compound B is treated with dilute sulfuric acid, compound C is obtained, which has molecular formula C7H160. Draw the structures of compounds A, B, and C.arrow_forwardCompound A is a derivative of the carbohydrate perosamine, which is found in the antibiotic perimycin. When A is treated with acetic anhydride in methanol, a monoacyl derivative B (C9H17NO5) is obtained in 73% yield. What is the structure of compound B?arrow_forward(a) 11. (b) What is the major product of the elimiation below? |||-- EtONa EtOH (C) (d)arrow_forward
- c) An alkyl halide E, C5H11B1 undergoes hydrolysis to form an alcohol F, C5H120. Alcohol F becomes cloudy immediately when reacted with a Lucas reagent. (i) (ii) Deduce the structures of E and F. State the type of reaction and propose the mechanism for the conversion of E to F. d) Draw the structure of the product formed when 2-bromopropane reacts with the following reagents respectively, (i) (ii) potassium cyanide ethoxide ionarrow_forwardShow how you would synthesize octan-2-one from each compound. You may use any necessary reagents.(a) heptanalarrow_forwardIdentify products A and B from the given 1H NMR data. Treatment of CH2=CHCOCH3 with one equivalent of HCl forms compound A. A exhibits the following absorptions in its 1H NMR spectrum: 2.2 (singlet, 3H), 3.05 (triplet, 2 H), and 3.6 (triplet, 2 H) ppm. What is the structure of A?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

