
Concept explainers
(a)
Interpretation:
The length of the face diagonal in terms of
Concept introduction:
The crystal can exist in various shapes but the most common one is cubic. The FCC unit cell has lattice points at eight corners of the unit cell along with the face centers of unit cell. The total number of atoms in FCC unit cell is four.
Answer to Problem 1ASA
The length of the face diagonal in terms of
Explanation of Solution
The face diagonal BC, represented by a, is shown below.
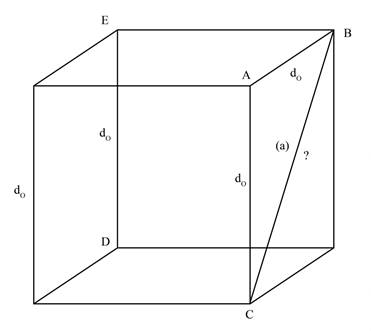
Figure 1
In the Figure 1, the face diagonal, represented by a, is the hypotenuse of right angle triangle ABC. The given unit cell is cube. Therefore, the length of AB will be equal to length of AC.
Therefore,
The length of face diagonal is calculated by using Pythagoras theorem as shown below.
Substitute the value of AB and AC in equation (1)
Therefore, the length of the face diagonal in terms of
The length of the face diagonal in terms of
(b)
Interpretation:
The length of the cube diagonal in terms of
Concept introduction:
The crystal can exist in various shapes but the most common one is cubic. The FCC unit cell has lattice points at eight corners of the unit cell along with the face centers of unit cell. The total number of atoms in FCC unit cell is four.
Answer to Problem 1ASA
The length of the cube diagonal in terms of
Explanation of Solution
A line that runs from one corner to other corner of the cube via centre of the cube is termed as cube diagonal. The cube diagonal is represented by CE as shown below.
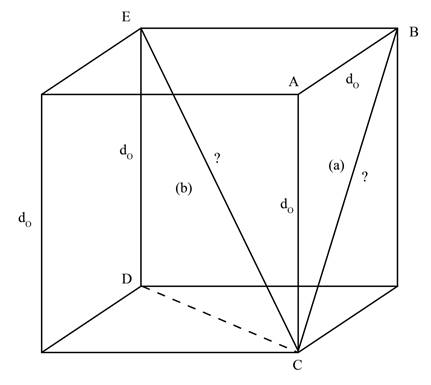
Figure 2
In the Figure 2, the cube diagonal, represented by b, is the hypotenuse of right angle triangle CDE. The edge length DE is given as
Therefore,
The length of face diagonal is calculated by using Pythagoras theorem as shown below.
Substitute the value of edge length DE and face diagonal CD in equation (2).
Therefore, the length of the cube diagonal in terms of
The length of the cube diagonal in terms of
Want to see more full solutions like this?
Chapter 16 Solutions
CHEMICAL PRIN.IN THE LAB-LM>CUSTOM<
- Consider the three types of cubic units cells. (a) Assuming that the spherical atoms or ions in a primitive cubic unit cell just touch along the cubes edges, calculate the percentage of occupied space within the unit cell. (Recall that the volume of a sphere is (4/3)r3, where r is the radius of the sphere.) (b) Compare the percentage of occupied space in the primitive cell (pc) with the bcc and fcc unit cells. Based on this, will a metal in these three forms have the same or different densities? If different, in which is it most dense? In which is it least dense?arrow_forwardAluminum metal crystallizes with a face-centered cubic unit cell. The volume of the cell is 0.0662 nm3. (a) What is the atomic radius of aluminum in cm? (b) What is the volume of a single aluminum atom? (c) What is the density of a single aluminum atom? (d) In face-centered cubic cell packing, the fraction of empty space is 26.0%. When this is factored in, what is the calculated density of aluminum?arrow_forwardPotassium iodide has a face-centered cubic unit cell of iodide ions with potassium ions in octahedral holes. The density of KI is 3.12 g/cm3. What is the length of one side of the unit cell? (Ion sizes are found in Figure 7.11.)arrow_forward
- You are given a small bar of an unknown metal X. You find the density of the metal to be 10.5 g/cm3. An X-ray diffraction experiment measures the edge of the face-centered cubic unit cell as 4.09 (1 = 1010 m). Identify X.arrow_forwardThe ionic radii of Cs+ and Cl are 181 and 167 pm, respectively. What is the length of the body diagonal in the CsCl unit cell? What is the length of the side of this unit cell? (CsCl has the same unit cell as CsI, shown in Figure 9.23.)arrow_forwardLike ZnS, lead(II) sulfide, PbS (commonly called galena), has a 1:1 empirical formula with a 2+ cation combined with the sulfide anion. Unit cell of PbS Sample of galena Does PbS have the same solid structure as ZnS? If different, how are they different? How is the unit cell of PbS related to its formula?arrow_forward
- The memory metal, nitinol, is an alloy of nickel and titanium. It is called a memory metal because after being deformed, a piece of nitinol wire will return to its original shape. The structure of nitinol consists of a simple cubic array of Ni atoms and an inner penetrating simple cubic array of Ti atoms. In the extended lattice, a Ti atom is found at the center of a cube of Ni atoms; the reverse is also true. a. Describe the unit cell for nitinol. b. What is the empirical formula of nitinol? c. What are the coordination numbers (number of nearest neighbors) of Ni and Ti in nitinol?arrow_forwardA diamond unit cell is shown here. Unit cell of diamond (a) How many carbon atoms are in one unit cell? (b) The unit cell can be considered as a cubic unit cell of C atoms with other C atoms in holes in the lattice. What type of unit cell is this (pc, bcc, fcc)? In what holes are other C atoms located, octahedral or tetrahedral holes?arrow_forwardAssume X has a body-centered cubic lattice with all atoms at the lattice points. The edge length of the unit cell is 379.0 pm. The atomic mass of X is 195.0 amu. Calculate the density of X.arrow_forward
- Vanadium metal has a density of 6.11 g/cm3. Assuming the vanadium atomic radius is 132 pm, is the vanadium unit cell primitive cubic, body-centered cubic, or face-centered cubic?arrow_forwardTitanium metal has a body-centered cubic unit cell. The density of titanium is 4.50 g/cm3. Calculate the edge length of the unit cell and a value for the atomic radius of titanium. (Hint: In a body-centered arrangement of spheres, the spheres touch across the body diagonal.)arrow_forwardTungsten crystallizes in a body-centered cubic unit cell with an edge length of 3.165 . (a) What is the atomic radius of tungsten in this structure? (b) Calculate the density of tungsten.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





