
Interpretation:
The carbonyl group of each of the eight
Concept Introduction:
Reduction Reaction: The
Example:
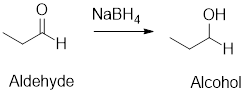
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
Optical activity: molecule or substances that can rotate the plane-polarized light are said to be optically active.
Meso compound are optically inactive, because meso compound is a stereoisomer with an identical or superimposable mirror image.
Plane of symmetry: When structure of the compound can be cut into two equal halves along the plane. Then such plane is called as plane of symmetry.
The stereoisomerism is the arrangement of atoms in molecules whose connectivity remains the same but their arrangement in different in each isomer.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Essential Organic Chemistry (3rd Edition)
- One of the later steps in glucose biosynthesis is the isomerization of fructose 6-phosphate to glucose 6-phosphate. Propose a mechanism, using acid or base catalysis as needed.arrow_forwardThe first step in the metabolism of glycerol, formed by digestion of fats, is phosphorylation of the pro-R—CH2OH group by reaction with adenosine triphosphate (ATP) to give the corresponding glycerol phosphate plus adenosine diphosphate (ADP). Show the stereochemistry of the product.arrow_forwardFollowing is a retrosynthesis for the coronary vasodilator ganglefene. (a) Propose a synthesis for ganglefene from 4-hydroxybenzoic acid and 3-methyl-3-buten-2-one. (b) Is ganglefene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forward
- The formation of Br2 from NBS first involves the reaction of NBS with HBr to form an iminol intermediate and molecular bromine. The intermediate then undergoes acid-catalyzed tautomerism to form succinimide, the byproduct of the reaction. Propose a curved-arrow mechanism for the conversion of NBS into succinimide that also accounts for the formation of Br2.arrow_forwardTreatment of -D-glucose with methanol in the presence of an acid catalyst converts it into a mixture of two compounds called methyl glucosides (Section 25.3A). In these representations, the six-membered rings are drawn as planar hexagons. (a) Propose a mechanism for this conversion and account for the fact that only the OH on carbon 1 is transformed into an OCH3 group. (b) Draw the more stable chair conformation for each product. (c) Which of the two products has the chair conformation of greater stability? Explain.arrow_forwardFats can be either optically active or optically inactive, depending on their structure. Draw the structure of an optically active fat that yields 2 equivalents of stearic acid and 1 equivalent of oleic acid on hydrolysis. Draw the structure of an optically inactive fat that yields the same products.arrow_forward
- What is the E2 major product of the structure?arrow_forwardrank the products in the indicated CH2 groups in order of increasing acidity and give reasons why.arrow_forwardThe rate constant for the uncatalyzed reaction of two molecules of glycine ethyl ester to form glycylglycine ethyl ester is 0.6 M- 1s - 1. In the presence of Co2 +, the rate constant is 1.5 * 106 M- 1s - 1. What rate enhancement does the catalyst provide?arrow_forward
- A) Describe substrate structure and condition that favor SN1 reactions taking place over SN2. B) What conditions promote E2 reactions.arrow_forwardImidazoleglycerol‑phosphate dehydratase is an enzyme in the histine biosynthesis pathway. It catalyzes the E1 dehydration of D‑erthyro‑imidazole‑glycerol phosphate to imidazole acetol‑phosphate. This is a rare example of a biological E1 reaction, as most biological elimination reactions occur through E1cB instead. In this reaction, D‑erthyro‑imidazole‑glycerol phosphate is first protonated to form a good leaving group. Then, the leaving group is ejected to form the resonance‑stabilized carbocation shown. Draw curved arrows forming the most stable resonance structure to explain why this reaction goes through an E1 mechanism. Draw curved arrows to form the most stable resonance structure.arrow_forwardtrehalose is a disacharide that can be obtained from fungi sea uchins and insects. acid hydrolysis of trehalose yields only D-glucose. trehalose is hydrolysed by a-glucosidase but not b-glucosidase.methylation of trhalose followed by hydrolysis yield two molar equivalents of 2-3-4-6 -tetra-O-methyl-D-glucopyranose. deduce the structure of the trehalose using the experimental dataarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


