
Basic Chemistry
6th Edition
ISBN: 9780134878119
Author: Timberlake, Karen C. , William
Publisher: Pearson,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 16, Problem 51UTC
Interpretation Introduction
Interpretation:
The new nucleus after the emission of a positron needs to be determined.
Concept introduction:
A proton is turned into a neutron and a positron (with a positive charge) leaves the nucleus.
Positron emission happens when a nucleus emits a positron
Expert Solution & Answer
Answer to Problem 51UTC
Solution:
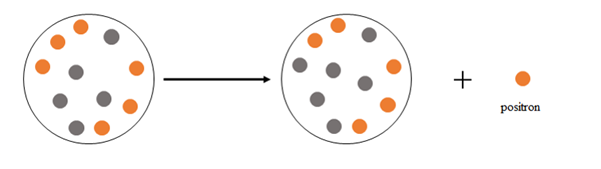
Explanation of Solution
Positron emission happens when a nucleus emits a positron
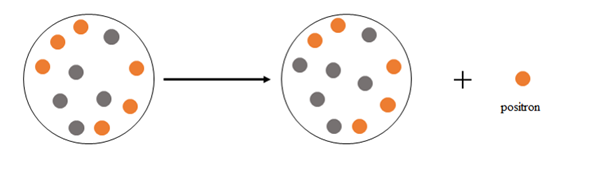
At the beginning there are 6 protons and 5 neutrons, after the emission there are 5 protons and 6 neutrons.
Conclusion
Thus, positron emission happens when a nucleus emits a positron
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
A sealed flask contains saturated sodium iodide with excess solid at the bottom of the flask. If a sample of radioactively labelled NaI(s) is added to the system, the most likely observation after 24 hours will be
Select one:
A.
neither the NaI(s) nor the only the NaI(aq) will register as radioactive
B.
both the NaI(s) and the NaI(aq) will register as radioactive
C.
only the NaI(aq) will register as radioactive
D.
only the NaI(s) will register as radioactive
Use the following information to answer the next question.
Crystals of magnesium phosphate and glucose, C6H12O6(s), were dissolved together in a beaker of distilled water.
All the entities that will be present when the two compounds are added to water are
Select one:
A.
Mg2+(s), PO43-(s), C6H12O6(aq), H2O(l)
B.
Mg2+(aq), PO43-(aq), C6H12O6(s), H2O(l)
C.
Mg3(PO4)2(aq), C6H12O6(s), H2O(l)
D.
Mg3(PO4)2(s), C6H12O6(aq), H2O(l)
Fluorine-18, which has a half-life of 110 min, is used in PET scans. If 129 mg of fluorine-18 is shipped at 8:00 a.m., how many milligrams of the radioisotope are still active if the sample arrives at the radiology laboratory at 1:30 p.m.? (enter answer with one place after the decimal)
For intitial radioactivity, 1 is the highest and 4 is the lowest
Chapter 16 Solutions
Basic Chemistry
Ch. 16.1 - Prob. 1PPCh. 16.1 - Prob. 2PPCh. 16.1 - Naturally occurring potassium consists of three...Ch. 16.1 - Prob. 4PPCh. 16.1 - Prob. 5PPCh. 16.1 - Prob. 6PPCh. 16.1 - Prob. 7PPCh. 16.1 - Prob. 8PPCh. 16.1 - Supply the missing information in the following...Ch. 16.1 - Prob. 10PP
Ch. 16.1 - Prob. 11PPCh. 16.1 - Prob. 12PPCh. 16.2 - Prob. 13PPCh. 16.2 - Prob. 14PPCh. 16.2 - Prob. 15PPCh. 16.2 - Prob. 16PPCh. 16.2 - Prob. 17PPCh. 16.2 - Prob. 18PPCh. 16.2 - Prob. 19PPCh. 16.2 - Prob. 20PPCh. 16.2 - Prob. 21PPCh. 16.2 - Prob. 22PPCh. 16.3 - Prob. 23PPCh. 16.3 - Prob. 24PPCh. 16.3 - Prob. 25PPCh. 16.3 - Prob. 26PPCh. 16.3 - Prob. 27PPCh. 16.3 - Prob. 28PPCh. 16.4 - Prob. 29PPCh. 16.4 - Prob. 30PPCh. 16.4 - Prob. 31PPCh. 16.4 - Prob. 32PPCh. 16.4 - Prob. 33PPCh. 16.4 - Prob. 34PPCh. 16.5 - Prob. 35PPCh. 16.5 - Prob. 36PPCh. 16.5 - Prob. 37PPCh. 16.5 - Prob. 38PPCh. 16.5 - Prob. 39PPCh. 16.5 - Prob. 40PPCh. 16.6 - Prob. 41PPCh. 16.6 - Prob. 42PPCh. 16.6 - Prob. 43PPCh. 16.6 - Prob. 44PPCh. 16.6 - Prob. 45PPCh. 16.6 - Prob. 46PPCh. 16.6 - Prob. 47PPCh. 16.6 - Prob. 48PPCh. 16.6 - Prob. 49PPCh. 16.6 - Prob. 50PPCh. 16 - Prob. 51UTCCh. 16 - Prob. 52UTCCh. 16 - The chapter sections to review are shown in...Ch. 16 - Prob. 54UTCCh. 16 - Prob. 55UTCCh. 16 - Prob. 56UTCCh. 16 - Prob. 57APPCh. 16 - Prob. 58APPCh. 16 - Prob. 59APPCh. 16 - Prob. 60APPCh. 16 - Prob. 61APPCh. 16 - Prob. 62APPCh. 16 - Prob. 63APPCh. 16 - Prob. 64APPCh. 16 - Prob. 65APPCh. 16 - Prob. 66APPCh. 16 - Prob. 67APPCh. 16 - Prob. 68APPCh. 16 - Prob. 69APPCh. 16 - Prob. 70APPCh. 16 - Prob. 71APPCh. 16 - Prob. 72APPCh. 16 - Prob. 73APPCh. 16 - Prob. 74APPCh. 16 - Prob. 75APPCh. 16 - Prob. 76APPCh. 16 - Prob. 77APPCh. 16 - Prob. 78APPCh. 16 - Prob. 79CPCh. 16 - Prob. 80CPCh. 16 - Prob. 81CPCh. 16 - Prob. 82CPCh. 16 - Prob. 83CPCh. 16 - Prob. 84CPCh. 16 - Prob. 85CPCh. 16 - Prob. 86CPCh. 16 - Prob. 87CPCh. 16 - Prob. 88CPCh. 16 - Prob. 89CPCh. 16 - Prob. 90CPCh. 16 - Consider the reaction of sodium oxalate (Na2C2O4)...Ch. 16 - Prob. 34CICh. 16 - Prob. 35CICh. 16 - Prob. 36CICh. 16 - Prob. 37CICh. 16 - Prob. 38CICh. 16 - Prob. 39CICh. 16 - Prob. 40CI
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- co-60 is used for radiation therapy as implants and as external source of radiation exposure. The half life of Co-60 is 5,272 years. how much of a 2.000 mg sample will remain after 21,088 years? Please show work.arrow_forwardStrontium-90 is a dangerous by-product of atomic testing because it mimics the action of calcium in the body. It decay in two B-emissions to give zirconium-90. Write a balanced nuclear equation for the overall decay of Sr-90. Calculate ◇m in grams when one mole of Sr-90 decays to Zr-90. How much energy (in KJ) is given off by the decay of 6.50 mg of Sr-90?arrow_forward- Give reason for 5- Isotopes have ability to undergo nuclear reactions which are very important in medical applications. 6- Radiation source in AAS device is hallow cathode lamp. 7- The PH of a solution Mg(OH)2 can affect a salt solubility by addition of H+ ion , pH decreasing while solubility of Mg(OH)2 will be increasing. * Short answer if you can ❤️*arrow_forward
- In the electron capture of In-110 A. The In-110 and the electron placed in the reactant side while the Cd-110 in the product side. B. The In-110 is placed in the reactant side while the Cd-110 and the electron in the product side. C. The Cd-110 and electron are placed in the reactant side while the In-110 in the product side. D. The Cd-110 is placed in the reactant side while the In-110 and the electron in the product side.arrow_forwardWhat is the energy change when the temperature of 14.0 grams of gaseous helium is decreased from 38.7 °C to 20.2 °C ?arrow_forwardIs -0.05 or 0.2 closer to 0 ?arrow_forward
- If a match contained 0.265 g of P4S3, what would be the energy released in kJ?arrow_forwardWhich has the LEAST radioactive carbon component? a. present plants b. crude oil c. whisky d. wine *Choose only one answer and explain.arrow_forwardStrontium-90 is a dangerous by-product of atomic testing because it mimics the action of calcium in the body. It decay in two β-emissions to give zirconium-90 (nuclear mass = 89.8824 g).a) Write a balanced nuclear equation for the overall decay of Sr-90.b) Calculate Δm in grams when one mole of Sr-90 decays to Zr-90.c) How much energy (in KJ) is given off by the decay of 6.50 mg of Sr-90?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning