
Figure 16.15 shows a pV diagram for a
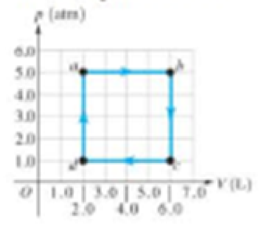
Figure 16.15
Problem 6.
Trending nowThis is a popular solution!
Learn your wayIncludes step-by-step video

Chapter 16 Solutions
COLLEGE PHYS. V.2 W/MOD.MAST. >LLF< >I
Additional Science Textbook Solutions
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics (4th Edition)
The Cosmic Perspective
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Life in the Universe (4th Edition)
College Physics
Essential University Physics: Volume 1 (3rd Edition)
- One process for decaffeinating coffee uses carbon dioxide ( M=44.0 g/mol) at a molar density of about 14,0 mol/m3 and a temperature of about 60 . (a) Is CO2 a solid, liquid, gas, or supercritical fluid under those conditions? (b) The van der Waals constants for carbon dioxide are a=0.3658 Pa m6/mol2 and b=4.286105 m3/mol. Using the van der Waals equation, estimate pressure of CO2 at that temperature and density. `arrow_forwardThe fpicture shows a pV diagram for an ideal gas in which its pressure tripled from a to b when 804 J of heat was put into the gas. a) How much work was done on or by the gas between aa and bb? Express your answer in joules. b) Fill in the sentence, do they increase, decrease, or stay the same. " The temperature of this gas ______ between a and b. When pressure increases, the temperature _____ provided that V and n are _______." c) By how much did the internal energy of the gas change between a and b? Express your answer in joules. Did it increase or decrease? d) What is the temperature of the gas at point b in terms of its temperature at a, Taarrow_forwardA sample containing 1.0 mole of oxygen gas initially at 45 degrees Celsius is heated to 110 degrees Celsius under isobaric conditions. From these given data, answer the questions that follows. What is the sample's change in internal energy (in KiloJoules)? What is the sample's change in enthalpy (in KiloJoules)?arrow_forward
- 2 moles of a monatomic ideal gas undergoes a cyclic process as depicted in the figure below. The processes AB and CD are isobaric and the process DA is adiabatic. For the given values PA= 11.5 atm, VA= 5.7 L, V3= 2.85 L, Pc=34.5 atm, and Vc=1.476 L answer the following questions. (use R=8.314 J 1 atm = 1.013x105 Pa, 1 L = 10-3 m³) . mol · K' Volume 1. Calculate the temperature TA= K 2. What type of process is the process BC? 3. Calculate the work done by the gas in the process DA. WDA = 4. Calculate the magnitude of the net heat entering the cycle. Q = 5. Calculate the magnitude of the net heat leaving the cycle. Qc = 6. Calculate the net work done by the gas. W= 7. Calculate the thermal efficiency of the cycle. e = % 8. Calculate the change in the entropy in the process AB. Include the sign (positive or negative) in your answer as well. ASAB = Karrow_forwardA gas expands from I to F in the figure below. The energy added to the gas by heat is 422 J when the gas goes from I to F along the diagonal path. Three paths are plotted on a PV diagram, which has a horizontal axis labeled V (liters), and a vertical axis labeled P (atm). The green path starts at point I (2,4), extends vertically down to point B (2,1), then extends horizontally to point F (4,1). The blue path starts at point I (2,4), and extends down and to the right to end at point F (4,1). The orange path starts at point I (2,4), extends horizontally to the right to point A (4,4), then extends vertically down to end at point F (4,1). (a) What is the change in internal energy of the gas? J(b) How much energy must be added to the gas by heat for the indirect path IAF to give the same change in internal energy? Jarrow_forward1 moles of a diatomic ideal gas undergoes a cyclic process as depicted in the figure below. The processes AB and CD are isobaric and the process DA is adiabatic. For the given values PA= 11.5 atm, VA= 6.5 L, V3= 3.25 L, Pc= 23 atm, and Vc=1.981 L answer the following questions. J (use R=8.314 1 atm = 1.013x105 Pa, 1 L= 10-3 m3) mol · K' Volume 1. Calculate the temperature TA K 2. What type of process is the process BC? 3. Calculate the work done by the gas in the process DA.WDA = 4. Calculate the magnitude of the net heat entering the cycle. |QH|=| 5. Calculate the magnitude of the net heat leaving the cycle. |Qcl = 6. Calculate the net work done by the gas. EW= 7. Calculate the thermal efficiency of the cycle. e = 8. Calculate the change in the entropy in the process AB. Include the sign (positive or negative) in Pressurearrow_forward
- An ideal monatomic gas is taken through the cycle in the PV diagram. where V1 = 1.20, V2 = 2.40, P1 = 98.0 kPa and P2 = 230 kPa. What is the change in internal energy of the gas as it is taken from A to B? How much work is done on this gas per cycle? What is the total change in internal energy of this gas in one cycle?arrow_forwardA container is filled with an ideal diatomic gas to a pressure and volume of P₁ and V₁, respectively. The gas is then warmed in a two-step process that increases the pressure by a factor of four and the volume by a factor of five. Determine the amount of energy transferred to the gas by heat if the first step is carried out at constant volume and the second step at constant pressure. (Use any variable or symbol stated above as necessary.) Q =arrow_forwardA container is filled with an ideal diatomic gas to a pressure and volume of P1 and V1, respectively. The gas is then warmed in a two-step process that increases the pressure by a factor of two and the volume by a factor of three. Determine the amount of energy transferred to the gas by heat if the first step is carried out at constant volume and the second step at constant pressure. (Use any variable or symbol stated above as necessary.)arrow_forward
- Converting sunlight to electricity with solar cells has an efficiency of 15%. It's possible to achieve a higher efficiency (though currently at higher cost) by using concentrated sunlight as the hot reservoir of a heat engine. Each dish in (Figure 1) concentrates sunlight on one side of a heat engine, producing a hot-reservoir temperature of 560 ∘C. The cold reservoir, ambient air, is approximately 30 ∘C. The actual working efficiency of this device is 30%. What is the theoretical maximum efficiency?arrow_forwardA gas expands from I to F in the figure below. The energy added to the gas by heat is 276 J when the gas goes from I to F along the diagonal path. A pressure-volume graph consists of points and line segments plotted on a coordinate plane, where the horizontal axis is V (liters)and the vertical axis is P (atm). Three points are plotted: point I at (2, 4) point A at (4, 4) point F at (4, 1) Line segments connect the three points to form a triangle. Arrows along the line segments point from I to A, from A to F, and from I to F. (a) What is the change in internal energy of the gas? J (b) How much energy must be added to the gas by heat along the indirect path IAF?arrow_forwardA system consisting of 0.0816 moles of a diatomic ideal gas is taken from state A to state C along the path in the figure below. (b) What is the lowest temperature of the gas during this process? In kelvin. (c) Find the change in internal energy of the gas in going from A to C. Hint: Adapt the equation (for the change in internal energy of a monatomic ideal gas) ΔU=3/2nRΔT=3/2Δ(PV)=3/2(PcVc-PaVa) to a diatomic ideal gas. In joules. (d) Find the energy delivered to the gas in going from A to C. In joules.arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

