
Concept explainers
(a)
Interpretation:
The products should be identified by the reaction of benzaldehyde with
Concept Introduction:
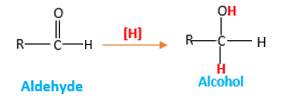
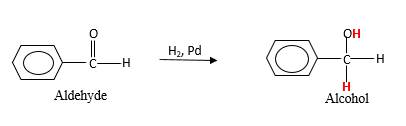
Addition of H2gas to a multiple bond is known as hydrogenation. In the presence of palladium metal as the catalyst H2 molecules react with
Answer to Problem 75P
Explanation of Solution
When an aldehyde reacts with H2 gas in the presence of palladium metal resulting product is an alcohol of the initial aldehyde molecule. Palladium metal act as a catalyst to the reaction that provides a surface to bind both the H2 and carbonyl compound which reduce the activation energy of the reaction.
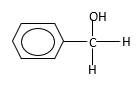
Hydrogen atoms in the alcohol molecule shown below, which are indicated in red color are the added H during the hydrogenation reaction.
(b)
Interpretation:
The products should be identified by the reaction of benzaldehyde with
Concept Introduction:
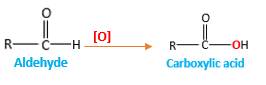
Addition of an O atom in to a molecule is known as oxidation. The hydrogen atom directly connected to the carbonyl C in an aldehyde will oxidized in the presence of an oxidizing agent such as
Oxygen atom in the carboxylicacid molecule shown below, which is indicated in red color is the added O during the oxygenation reaction.
Answer to Problem 75P
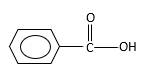
Explanation of Solution
When an aldehyde reacts with
(c)
Interpretation:
The products should be identified by the reaction of benzaldehyde with
Concept Introduction:
Addition of an O atom in to a molecule is known as oxidation. The hydrogen atom directly connected to the carbonyl C in an aldehyde will oxidized in the presence of an oxidizing agent such as (
Oxygen atom in the carboxylic acid molecule shown below, which is indicated in red color is the added O during the oxygenation reaction.
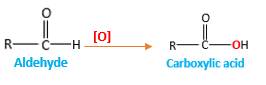
Answer to Problem 75P
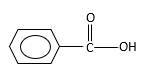
Explanation of Solution
When an aldehyde reacts with
Aldehyde's
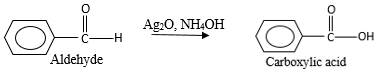
(d)
Interpretation:
The products should be identified by the reaction of benzaldehyde with
Concept Introduction:
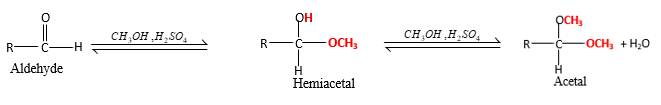
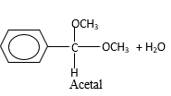
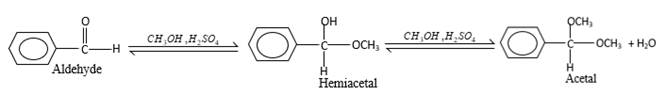
In the presence of alcohol in the acidic medium, aldehydes undergo addition reactions and give acetal in two steps.
Hydrogen atom and CH3 groups in the acetal and hemiacetal molecules shown below, which are indicated in red color are the added molecules during the reaction.
Answer to Problem 75P
Explanation of Solution
In the presence of alcohol in the acidic medium, aldehydes undergo addition reactions and give acetal in two steps. In the first step aldehydes form hemiacetals and during the second step it converts to an acetal molecule of the respective aldehyde molecule.
Addition of one molecule of alcohol in to an aldehyde forms a hemiacetal, one bond of the
(e)
Interpretation:
The products should be identified by the reaction of benzaldehyde with
Concept Introduction:
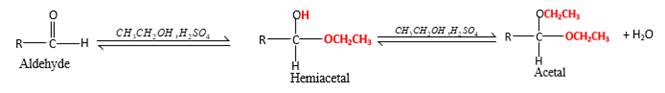
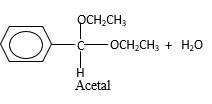
In the presence of alcohol in the acidic medium, aldehydes undergo addition reactions and give acetal in two steps.
Hydrogen atom and CH2CH3 groups in the acetal and hemiacetal molecules shown below, which are indicated in red color are the added molecules during the reaction.
Answer to Problem 75P
Explanation of Solution
In the presence of alcohol in the acidic medium, aldehydes undergo addition reactions and give acetal in two steps. In the first step aldehydes form hemiacetals and during the second step it converts to an acetal molecule of the respective aldehyde molecule.
Addition of one molecule of alcohol in to an aldehyde forms a hemiacetal, one bond of the
(f)
Interpretation:
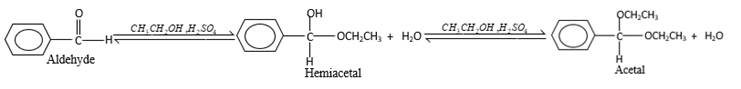
The products should be identified by the reaction of
Concept Introduction:
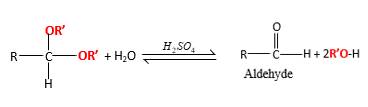
In the presence of water and acid, acetals undergo hydrolysis reaction and produce aldehydes.
OR' groups in the acetal molecule shown below, which are indicated in red color are the molecules which becomes alcohol molecules during the hydrolysis.
Answer to Problem 75P
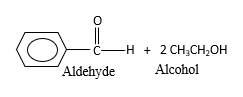
Explanation of Solution
Acetals are stable molecules, but their bonds can cleave by a reaction with water and produce aldehydes.
In the acetal molecule, two bonds of the
Want to see more full solutions like this?
Chapter 16 Solutions
GENERAL, ORGANIC & BIOLOGICAL CHEMISTRY
- Draw the missing starting material. Reagent 1 is benzene and AlCl3. Reagent B is Zn(Hg) and HCl.arrow_forwardWhat starting materials are needed to prepare each compound using a Heckreaction?arrow_forward1. O-hydroxybenzoic acid is a major product formed with phenol and which other reactant/s I-primary alcohol II-sodium hydroxide III-water IV-carbon dioxide A.I and III B. I and IV C. II and III D. II and IVarrow_forward
- Draw the organic products formed when allylic alcohol A is treated with each reagent.a.H2 + Pd-C b.mCPBA c. PCC d.CrO3, H2SO4, H2O e.(CH3)3COOH, Ti[OCH(CH3)2]4, (+)-DET f. (CH3)3COOH, Ti[OCH(CH3)2]4, (−)-DET g. [1] PBr3; [2] LiAlH4; [3] H2O h.HCrO4−–Amberlyst A-26 resinarrow_forwardDraw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. d. (CH3CH2)2CHCOCl, AlCl3 j. product in (d), then NH2NH2, – OHarrow_forward1. Which compound is more soulable in water : CH3 CH2 CH2 CH2 CH2 CH3 OR CH3 CH2 CH2 CH2 COOH 2. Which compound is more soulable in organic solvent : CH3 CH2 CH2 CH2 CH2 CH3 OR CH3 CH2 CH2 CH2 COOHarrow_forward
- Amino acids such as glycine are the building blocks of large molecules called proteins that give structure to muscle, tendon, hair, and nails. a. Explain why glycine does not actually exist in the form with all atoms uncharged, but actually exists as a salt called a zwitterion. b. What product is formed when glycine is treated with concentrated HCl? c. What product is formed when glycine is treated with NaOH?arrow_forwardDraw the products formed when p-methylaniline (p-CH3C6H4NH2) is treated with each reagent. a. HCl b. CH3COCl c. (CH3CO)2O d. excess CH3I e. (CH3)2C = O f. CH3COCl, AlCl3 g. CH3CO2H h. NaNO2, HCl i. Part (b), then CH3COCl, AlCl j. CH3CHO, NaBH3CNarrow_forward1. Write the skeletal structures of propanal, acetone and cyclohexanone. What is the major intermolecular force (IMF) found in them? Based on their major intermolecular force and molecular weight, what can you predict on their solubility in water? Chemical Name Skeletal Structures Major IMF Solubility in water Propanal Acetone Cyclohexanone 2. What is the purpose of Tollens’ test (Part B)? What is the evidence of a positive result? 3. What is the purpose of oxidation test (Part C)? What is the evidence of a positive result?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
