
Introduction To General, Organic, And Biochemistry
12th Edition
ISBN: 9781337571357
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 11P
18-14 Answer true or false.
(a) Carboxylic acids are polar compounds.
(b) The most polar bond of a carboxyl group is the C—O single bond.
(c) Carboxylic acids have signi?cantly higher boiling points than
(d) The low-molecular-weight carboxylic acids (formic, acetic, propanoic, and butanoic acids) are in?nitely soluble in water.
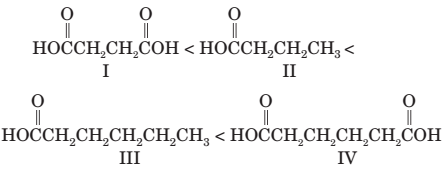
(e) The following compounds are arranged in order of increasing boiling point:
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 17 Solutions
Introduction To General, Organic, And Biochemistry
Ch. 17.2 - Prob. 17.1QCCh. 17.5 - Prob. 17.2QCCh. 17.5 - Prob. 17.3QCCh. 17 - 18-4 Answer true or false. (a) The functional...Ch. 17 - Prob. 2PCh. 17 - 18-6 Name and draw structural formulas for the...Ch. 17 - 18-7 Write the IUPAC name for each carboxylic...Ch. 17 - 18-8 Write the IUPAC name for each carboxylic...Ch. 17 - Prob. 6PCh. 17 - Prob. 7P
Ch. 17 - Prob. 8PCh. 17 - Prob. 9PCh. 17 - Prob. 10PCh. 17 - 18-14 Answer true or false. (a) Carboxylic acids...Ch. 17 - 18-15 Draw a structural formula for the dimer...Ch. 17 - 18-16 Propanedioic (malonic) acid forms an...Ch. 17 - 18-17 Hexanoic (caproic) acid has a solubility in...Ch. 17 - 18-18 Propanoic acid and methyl acetate are...Ch. 17 - 18-19 The following compounds have approximately...Ch. 17 - Prob. 17PCh. 17 - Prob. 18PCh. 17 - Prob. 19PCh. 17 - 18-23 Characterize the structural features...Ch. 17 - Prob. 21PCh. 17 - Prob. 22PCh. 17 - 18-26 Answer true or false. (a) Carboxylic acids...Ch. 17 - Prob. 24PCh. 17 - 18-28 Arrange these compounds in order of...Ch. 17 - 18-29 Complete the equations for these acid—base...Ch. 17 - 18-30 Complete the equations for these acid-base...Ch. 17 - 18-31 Formic acid is one of the components...Ch. 17 - Prob. 29PCh. 17 - Prob. 30PCh. 17 - Prob. 31PCh. 17 - Prob. 32PCh. 17 - Prob. 33PCh. 17 - Prob. 34PCh. 17 - 18-38 Which is the stronger base: CH3CH2NH2 or...Ch. 17 - Prob. 36PCh. 17 - Prob. 37PCh. 17 - 18-41 Complete these examples of Fischer...Ch. 17 - Prob. 39PCh. 17 - Prob. 40PCh. 17 - Prob. 41PCh. 17 - Prob. 42PCh. 17 - 18-46 Procaine (its hydrochloride salt is marketed...Ch. 17 - 18-47 Methylparaben and propylparaben are used as...Ch. 17 - 18-48 4-Aminobenzoic acid is prepared from benzoic...Ch. 17 - Prob. 46PCh. 17 - Prob. 47PCh. 17 - Prob. 48PCh. 17 - Prob. 49PCh. 17 - Prob. 50PCh. 17 - Prob. 51PCh. 17 - Prob. 52P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 18-19 The following compounds have approximately the same molecular weight: hexanoic acid, heptanal, and 1-heptanol. Arrange them in order of increasing boiling point.arrow_forward18-6 Name and draw structural formulas for the four carboxylic acids with the molecular formula C5H10O2. Which of these carboxylic acids are chiral?arrow_forward16-54 Several poisonous plants, including Atropa belladonna, contain the alkaloid atropine. The name “belladonna” (which means “beautiful lady”) probably comes from the fact that Roman women used extracts from this plant to make themselves more attractive. Atropine is widely used by ophthal mologists and optometrists to dilate the pupils for eye examination. Classify the amino group in atropine as primary, secondary, or tertiary. Locate all stereocenters in atropine. Account for the fact that atropine is almost insoluble in water (1 g in 455 mL of cold water) but atropine hydrogen sulfate is very soluble (1 g in 5 mL of cold water). Account for the fact that a dilute aqueous solution of atropine is basic (pH approximately 10.0).arrow_forward
- 18-41 Complete these examples of Fischer esterification. In each case, assume an excess of the alcohol.arrow_forward18-18 Propanoic acid and methyl acetate are constitutional isomers, and both are liquids at room temperature. One of these compounds has a boiling point of 141°C; the other has a boiling point of 57°C. Which compound has which boiling point? Explain.arrow_forward17-70 What simple chemical test could you use to distinguish between the members of each pair of com pounds? Tell what you would do, what you would expect to observe, and how you would interpret your experimental observation. (a) Benzaldehyde and cyclohexanone (b) Acetaldehyde and acetonearrow_forward
- 17-12 Is it possible for the carbon atom of a carbonyl group to be a stereocenter? Explain.arrow_forward17-26 Account for the fact that acetone has a higher boiling point (56°C) than ethyl methyl ether (11°C) even though their molecular weights are almost the same.arrow_forward2 (Chemical Connections 19A) Locate the ester group in pyrethrin I and draw a structural formula for chrysanthemic acid, the carboxylic acid from which this ester is derived.arrow_forward
- 17-69 Propanal (bp 49°C) and 1-propanol (bp 97°C) have about the same molecular weight, yet their boiling points differ by almost 50°C. Explain this fact.arrow_forward18-28 Arrange these compounds in order of increasing acidity: benzoic acid, benzyl alcohol, phenol.arrow_forward7 What type of structural feature do the anhydrides of phosphoric acid have in common with carboxylic acids?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY