
Concept explainers
Draw the predominant form of the following amino acids at physiological pH (7.4):
- a. Lysine
- b. Arginine
- c. tyrosine
(a)
Interpretation:
The predominant form of lysine at physiological
Concept introduction:
Acid-base properties of Amino acids:
Every amino acid has a carbonyl group and an amino group, and each group can exist in an acidic form or a basic form, depending on
The compound exists primarily in the acidic form in solutions that are more acidic than their
At
Answer to Problem 29P
The predominant form of lysine at physiological
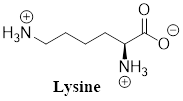
Explanation of Solution
The predominant form of glutamine at physiological

(b)
Interpretation:
The predominant form of arginine at physiological
Concept introduction:
Acid-base properties of Amino acids:
Every amino acid has a carbonyl group and an amino group, and each group can exist in an acidic form or a basic form, depending on
The compound exists primarily in the acidic form in solutions that are more acidic than their
At
Answer to Problem 29P
The predominant form of lysine at physiological
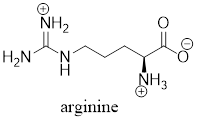
Explanation of Solution
The predominant form of lysine at physiological
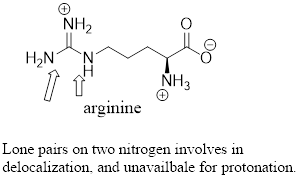
(c)
Interpretation:
The predominant form of tyrosine at physiological
Concept introduction:
Acid-base properties of Amino acids:
Every amino acid has a carbonyl group and an amino group, and each group can exist in an acidic form or a basic form, depending on
The compound exists primarily in the acidic form in solutions that are more acidic than their
At
Answer to Problem 29P
The predominant form of tyrosine at physiological
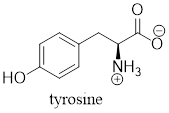
Explanation of Solution
The predominant form of tyrosine at physiological

Want to see more full solutions like this?
Chapter 17 Solutions
Essential Organic Chemistry, Global Edition
- Draw the predominant form the tyrosine amino acids at physiological pH (7.4):arrow_forwardExplain stereisomerism exist in ataraxarrow_forwardChemistry 1a. The lack of an asymmetric carbons and ease of rotation make glycine rich protein sequences: a. Fibrous b. Helical c. Cyclic d. Flexible e. Globular 1b. Introducing D-amino acids into peptides can be useful as a strategy to: a. Change pKa b. Reduce thermal stability c. Alter ionic strength d. Slow degradation e. Increase solubility 1c. Cellulose is not useful dietary carbohydrate because humans: a. Don’t have sufficient non-mitochondrial ATP b. Lack a 1,4-beta glycosidase c. Can’t digest branched chain carbohydrates d. Loose lactase as they mature e. Can’t absorb pentise subunitsarrow_forward
- Draw the structure of a naturally occurring amino acid that: a. contains a 1 ° alcohol b. contains an amide c. is an essential amino acid with an aromatic ring d. is a neutral amino acid with a 3 ° carbon atom in its side chainarrow_forwardPart A. Draw the peptide formed between asparagine and histidine. Part B Fill out the following tablearrow_forwardOdd-One-Out. Find which of the following is not included in the group. 36. a. Tryptophan b. Tyrosine c. Phenylalanine d. Histidine37. a. Arginine b. Histidine c. Tryptophan d. Lysine38. a. Alcohol b. Amide c. Basic d. Sulfur39. a. Alanine b. Glysine c. Methionine d. Leucine40. a. Glu, G b. Cys, C c. Val, V d. Pro, P41. a. Asp, D b. Tyr, T c. Lys, K d. Leu, Larrow_forward
- you have a 11.5 mg sample of blood that contain various proteins. Hemoglobin is only protein containing Fe. Hemoglobin is 3.3% of compund C34H32FeN4O4. 11.5 mg blood sample contains 34.7 micrograms of Fe. What is mass % of hemoglobin in protein sample?arrow_forwardEstimate the pI of each tripeptide in Problem 27.46. Draw a structural formula of these tripeptides. Mark each peptide bond, the N-terminal amino acid, and the C-terminal amino acid. (a) Phe-Val-Asn (b) Leu-Val-Glnarrow_forwardDraw the predominant form for each of the following amino acids at physiological pH (7.4) a. aspartate c. glutamine e. arginine b. histidine d. lysine f. tyrosinearrow_forward
- Calculate the pI value of GE- dipeptide The pKa values of the terminal amino and carboxyl groups are 8 and 3.5. Show all steps towards the answer.arrow_forwardC6H8O6(aq) + C12H7O2NCL2(aq) ----> C6H6O6(aq) + C12H9O2NCL2(aq) - C12H7O2NCL2 is abbreviated as DCIP - Molar Lass of C6H8O6(s) is 176.14 g/mol - Daily recommended intake (DRI) for adults: 90 mg of Vitamin C Askor & Bich found that it required 23.14 mlof 1.32 x 10^-3 M DCIP soliution to reach the endpoint when titrating a 7.50ml aliquot of pear juice. How many mg of ascorbic acid, C6H8O6 are present in the juice aliquot? _5.38_____(can you please show how to calculate this answer) How many ml of this juice would Bich have to drink in order to meet the daily recommended intake (DRI)?__126__ (arrow_forwardNet Charge on an Amino Group.The amino acid glycine has hydrogen as its side-chain (R) group. Values of pKca= 2:36 and pKaa=9:56 have been previously reported for glycine. Superscripts c, a, and s refer to a-carboxylic acid, α-amino base, and side chain, respectively. Using these values, determine the net charge on the a-amino group of glycine at a physiologic pH of 7.2 and a pH of 3 [70].arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning  Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



