
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 17, Problem 49P
Interpretation Introduction
Interpretation:
The difference in the
Concept introduction:
The value of
If the acidity or easiness of the protonation is high for a compound, then the value of the
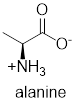
The structure of alanine is,
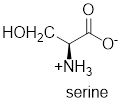
The structure of serine is,
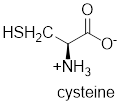
The structure of cysteine is,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain why the pI of lysine is the average of the pKa values of its two protonated amino groups.
1. Taking the structure of the amino acids into account, give a comparative discussion pK values where histidine=2 and alanine=2.
Taking the structure of the amino acids into account, give a comparative discussion of these pK values :
Alanine : 2.34
: 6.1
: 9.70
Histidine: 1.82
: 9.17
Chapter 17 Solutions
Essential Organic Chemistry, Global Edition
Ch. 17.1 - a. Explain why, when the imidazole ring of...Ch. 17.2 - Prob. 2PCh. 17.3 - Prob. 3PCh. 17.3 - Prob. 4PCh. 17.3 - Prob. 6PCh. 17.4 - Calculate the pI of each of the following amino...Ch. 17.4 - a. Which amino acid has the lowest pI value? b....Ch. 17.5 - What aldehyde is formed when valine is treated...Ch. 17.5 - Prob. 10PCh. 17.5 - Prob. 11P
Ch. 17.5 - Prob. 12PCh. 17.6 - Prob. 13PCh. 17.6 - What amino acid would be formed using the...Ch. 17.6 - What amino acid would be formed when the aldehyde...Ch. 17.7 - Pig liver esterase is an enzyme that catalyzes the...Ch. 17.8 - Prob. 17PCh. 17.8 - Prob. 18PCh. 17.8 - Prob. 19PCh. 17.8 - Prob. 20PCh. 17.10 - Prob. 21PCh. 17.10 - Prob. 22PCh. 17.10 - Why does cyanogen bromide not cleave on the C-side...Ch. 17.10 - Prob. 24PCh. 17.10 - Prob. 26PCh. 17.12 - Prob. 27PCh. 17.13 - a. Which would have the greatest percentage of...Ch. 17 - Draw the predominant form of the following amino...Ch. 17 - What is the pI of serine?Ch. 17 - Prob. 31PCh. 17 - Prob. 32PCh. 17 - Which would have a higher percentage of negative...Ch. 17 - Draw the form of aspartate that predominates at...Ch. 17 - Prob. 35PCh. 17 - A professor was preparing a manuscript for...Ch. 17 - a. Why is the pKa of the glutamate side chain...Ch. 17 - Prob. 38PCh. 17 - Determine the amino acid sequence of a polypeptide...Ch. 17 - Prob. 40PCh. 17 - Prob. 41PCh. 17 - Three peptides were obtained from a trypsin...Ch. 17 - Prob. 43PCh. 17 - After the polypeptide shown here was treated with...Ch. 17 - The disulfide bridges of a polypeptide were...Ch. 17 - -Amino acids can be prepared by treating an...Ch. 17 - Reaction of a polypeptide with carboxypeptidase A...Ch. 17 - Prob. 48PCh. 17 - Prob. 49PCh. 17 - Show how valine can be prepared by a. a Strecker...Ch. 17 - Prob. 51PCh. 17 - Why is proline never found in an -helix?Ch. 17 - Determine the amino acid sequence of a polypeptide...Ch. 17 - Prob. 55PCh. 17 - A chemist wanted to test his hypothesis that the...Ch. 17 - A normal polypeptide and a mutant of the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Alanine has pKa values of 2.34 and 9.69. Therefore, alanine exists predominately as a zwitterion in anaqueous solution with pH 7 ____ and pH 6 ____.arrow_forwardThe pKa of the amino and carboxyl groups on alanine are 9.9 and 2.4, respectively. Under physiological conditions (pH 7.4), what percentage of alanine is ionized?arrow_forwardAlanine has pKa values of 2.34 and 9.69. Therefore, alanine exists predominately as a zwitterion in an aqueous solution with pH > ____ and pH < ____.arrow_forward
- Draw the structures of the amino acids phenylalanine and aspartate in the ionization state you would expect at pH 7.0. Why is aspartate very soluble in water, whereas phenylalanine is much less soluble?arrow_forwardThe pKa values for the amino and carboxyl groups of alanine are 9.69 and 2.35, respectively. Calculate the pH of 10 mL of 50 mM alanine buffer (pH 10) following the addition of: a. 0.1 mL of 1.5 M HCl b. 0.3 mL of 1.5 M HClarrow_forwardThe configuration of the chiral center in a-amino acids is most commonly specified using the d,l convention. It can also be identified using the R,S convention . Does the chiral center in l-serine have the R or S configuration?arrow_forward
- At very low pH, alanine is a diprotic acid that can be represented as H3N1-CH(CH3)-COOH. The pKa of the carboxyl group is 2.3, and the pKa of the UNH1 3 group is 9.7.(a) At pH 7, what fraction of the amino acid molecules dissolved in an aqueous solution will have the form H3N1-CH(CH3)-COO2?(b) What fraction of the molecules at this pH will havethe form H2N-CH(CH3)-COOH?arrow_forwardGiven: Alanine: pKa=4 Pkb=9 Histidine: pKa=2 pKb=6 and pKc=9 Taking the structure of the amino acids into account, give a comparative discussion of these pK values.arrow_forwardDraw Tryptophan in a peptide bond. Explain all of the bonds using valence bond theory (VBT). Next, explain where VBT fails and how molecular orbital theory is a better description of key regions of this amino acid and the peptide bonds it forms with other residuesarrow_forward
- Valine has pKa's of 2.286 and 9.719. Estimate the fractional composition of Valine in the -1 form at pH=6.arrow_forward22-71 Which amino acid side chain is most frequently involved in denaturation by reduction?arrow_forwardFor lysine and arginine, the isoelectric point, pI, occurs at a pH where the net charge on the nitrogen-containing groups is +1 and balances the charge of -1 on the a-carboxyl group. Calculate pI for these amino acids.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning