
Concept explainers
(a)
The final gauge pressure of the tire.
(a)
Explanation of Solution
Given:
Initial gauge pressure is
Initial temperature of tire is
Final temperature of tire is
The atmospheric pressure is
Formula used:
Write the expression for ideal-
Rearrange the above expression in term of
Here,
Write the expression for gauge pressure.
Here,
Calculation:
Calculate the initial pressure of gas.
Convert the initial temperature from
Convert the final temperature from
Substitute
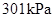 for
for
Substitute
Conclusion:
Thus, the final gauge pressure of tire is
(b)
The final gauge pressure of the tire.
(b)
Explanation of Solution
Given:
Initial gauge pressure is
Initial temperature of tire is
Final temperature of tire is
The atmospheric pressure is
The increase in volume is
Formula used:
Write the expression for ideal-gas law.
The initial and final volume of gas is equal.
Rearrange the above expression in term of
Here,
Write the expression for gauge pressure.
Here,
Calculation:
Calculate the initial pressure of gas.
Convert the initial temperature from
Convert the final temperature from
Calculate the final volume in term of initial volume.
Substitute
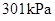 for
for
Substitute
Conclusion:
Thus, the final gauge pressure of tire is
Want to see more full solutions like this?
Chapter 17 Solutions
Physics for Scientists and Engineers, Vol. 3
- A company advertises that it delivers helium at a gauge pressure of 1.72107 Pa in a cylinder of volume 43.8 L. How many balloons can be inflated to a volume of 4.00 L with that amount of helium? Assume the pressure inside the balloons is 1.72105 Pa and the temperature in the cylinder and the balloons is 25.0 .arrow_forwardWhat is the total translational kinetic energy of the air molecules in a room of volume 23 m3 if the pressure is 9.5104 Pa (the room is at fairly high elevation) and die temperature is 21 ? Is any item of data unnecessary for the solution?arrow_forwardA container which is air tight has a volume of * 2 * 500L internal pressure of 1.0 atmosphere, internal temperature of 15 °C is washed off ythe deck of a ship located in the Philippine Trench, and sinks to a depth where the pressure is 175 atmosphere and the temperature is 3 °C. What would be the volume in m³ of the gas inside be when the container breaks under the pressure at this depth?arrow_forward
- The law of a confined ideal gas is P V = k T, where P is pressure, V is the volume, T (in Celsius) is the temperature and k > 0 is the constant. The gas is being heated at a rate of 2ºC/min and the pressure increases at a rate of 0.5 kgf/cm. minute. if in At a certain moment, the temperature is 200 degrees and the pressure is 10 kgf/cm2 , find the rate at which the volume changes for k = 8 answer:the volume describes the rate of 32/5 cm³/minarrow_forwardIf I have an unknown quantity of gas at a pressure of 1.2 atm, a volume of 31 liters, and a temperature of 87 degress celsius, how many moles of gas do I have?arrow_forwardA tire is filled with air at 15 degree C to a gauge pressure of 230kPa. If the tire reaches a temperature of 38 degree C, what fraction of the original air must be removed if the original pressure of 230 kPa is to be maintained?arrow_forward
- Suppose that an ideal gas in a sealed metallic metal container so that it has a fix volume has a temperature increase by a factor of 2.64x. By what factor with the pressure of the gas increase or decrease in the container?arrow_forwardThe gage pressure of an automobile tire is measured to be 200 kPa before a trip and 220 kPa after the trip at a location where the atmospheric pressure is 90 kPa. Assuming the volume of the tire remains constant at 0.035 m3, determine the percent increase in the absolute temperature of the air in the tire.arrow_forwardA container holds 2.5 moles of an ideal gas at a pressure of 3.5 atmospheres and a temperature of 300 Kelvin. If the volume of the container is 10 liters, what is the value of the gas constant (R) in J/(mol·K)?arrow_forward
- If a pneumatic cylinder has a volume of 14 cubic inches at 1000 PSIG when the temperature is 85°F and then the temperature is elevated to 125°F by compression to 12 cubic inches. What does the gage pressure increase to?arrow_forwardIf the pressure in a car tire is 2.0 atm at 27°C, what will be the pressure if the temperature warms to 57°C? Assume that the volume of the tire remains constant. A patient with heat stroke has a temperature of 105.8 0 What does this read on a Celsius thermometer?arrow_forwardTwo vessels X & Y of volumes 10x10-4 m3 and 5x10-4 m3 connected by a tube of negligible volume and kept at temperatures of 400 K and 200 K respectively, contain the same ideal gas. What is the ratio of nX : nY?arrow_forward

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning


