
Concept explainers
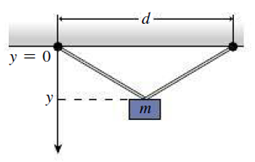
Figure 17.12 shows an apparatus used to determine the linear expansion coefficient of a metal wire. The wire is attached to two points a distance d apart (you don’t know d). A mass hangs from the middle of the wire. The wire’s total length is 100.00 cm at 0°C. The distance y from the suspension points to the top of the mass is measured, and the results are given in the table below. (a) Find an expression for v as a function of temperature, and manipulate your expression to get a linear relation between some function of y and some function of temperature T. You’ll encounter the expression L2 where L is the length of the wire, and, because the change in length is small, you can drop terms involving α2 when you expand L2. (b) Calculate the quantities in your relation from the given data, and plot. Determine a best-fit line and use it to determine the coefficient of linear expansion a and the separation d. (c) Consult Table 17.2 to identify the metal the wire is made of. Ignore any stretching of the wire due to its “springiness”; that is, consider only thermal expansion.
| Temperature. T (°C) | 0 | 20 | 40 | 60 | 80 | 100 | 120 |
| y (cm) | 30.00 | 30.05 | 30.07 | 30.11 | 30.16 | 30.19 | 30.24 |
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Essential University Physics
Additional Science Textbook Solutions
Life in the Universe (4th Edition)
Physics: Principles with Applications
The Cosmic Perspective
College Physics: A Strategic Approach (3rd Edition)
University Physics Volume 2
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics (4th Edition)
- Aluminum has a density of 2.712 ✕ 103 kg/m3 at 0°C and an average linear expansion coefficient of 2.200 ✕ 10−5(°C−1). A 15.00 kg block of aluminum at 0°C is heated to 70.00°C. (a)What is the density (in kg/m3) of the aluminum block at 70.00°C? (Round your answer to at least four significant figures.) Answer- kg/m3 (b)What is the mass (in kg) of the aluminum block at 70.00°C? Answer- kgarrow_forwardA hollow aluminum cylinder 15.5 cm deep has an internal capacity of 2.000 L at 22.0°C. It is completely filled with turpentine at 22.0°C. The turpentine and the aluminum cylinder are then slowly warmed together to 80.0°C. (The average linear expansion coefficient for aluminum is 24 ✕ 10−6°C−1, and the average volume expansion coefficient for turpentine is 9.0 ✕ 10−4°C−1.) How much turpentine overflows? 2. What is the volume of turpentine remaining in the cylinder at 80.0°C? (Give your answer to at least four significant figures.) 3. If the combination with this amount of turpentine is then cooled back to 22.0°C, how far below the cylinder's rim does the turpentine's surface recede?arrow_forwardA spherical steel ball bearing has a diameter of 2.540 cm at 26.00°C. (Assume the coefficient of linear expansion of the steel is 1.10 10-5(°C-1), and the average volume expansion coefficient is 3.30 10-5(°C-1).) A) What is its diameter when its temperature is raised to 97.0°C? (Give your answer to four significant figures in cm.) B) What temperature change is required to increase its volume by 1.100%?arrow_forward
- A metal tank with a capacity of 1700 L is completely filled with ethanol when both the tank and the ethanol are at temperature 19.0 ∘C. Then the tank and its contents are brought underground, where the temperature is 10.0 ∘C . What volume of air will there be above the ethanol in the tank after the system has cooled off to the ground temperature? The coefficient of volume expansion for ethanol is βe = 7.50×10−4 K−1 . The tank is made of a metal for which the coefficient of linear expansion is αt = 1.20×10−5 K−1. a) Find the change in volume of the metal tank, ΔVt, and that of the ethanol in it, ΔVe b What volume of air Vair will there be above the ethanol in the tank after the system has cooled off to the ground temperature?arrow_forwardIf I have an unknown quantity of gas held at a temperature of 365 K in a container with a volume of 25.9 L and a pressure of 8.5 atm, how many moles of gas do I have?arrow_forwardAn aluminum cup of 61 cm3 capacity is completely filled with glycerin at 22°C. How much glycerin will spill out of the cup if the temperature of both the cup and glycerin is increased to 41°C? (The linear expansion coefficient of aluminum is 23 × 10-6 1/C°. The coefficient of volume expansion of glycerin is 5.1 × 10-4 1/C°.)arrow_forward
- Most automobiles have a coolant reservoir to catch radiator fluid that may overflow when the engine is hot. A radiator is made of copper and is filled to its 14.2 L capacity when at 18.0°C. What volume (in L) of radiator fluid will overflow when the radiator and fluid reach their 95.0°C operating temperature, given that the fluid's volume coefficient of expansion is ? = 400 ✕ 10−6/°C? Note that this coefficient is approximate, because most car radiators have operating temperatures of greater than 95.0°C. _______________ Larrow_forwardA cylinder is closed by a piston connected to a spring of constant 2.40 x 103 N/m. With the spring relaxed, the cylinder is filled with 5.00 L of gas at a pressure of 1.00 atm and a temperature of 20.0°C. a) If the piston has a cross-sectional area of 0.0130 m 2 and negligible mass, how high (in meter) will it rise when the temperature is raised to 250°C? b) The pressure of the gas at 250°C = __ × 105 Paarrow_forwardA plastic bar’s length is 2.756 meters (m) and its temperature is 99.00 ̊C. When it is cooled, the temperature is 11.00 ̊C and length is 2.738 m. a)Find the coefficient of linear expansion of the bar. in units of ̊C−1. b)what is the length (in meters), if heated at 123.00 ̊C,arrow_forward
- A bridge has a span of 556.7 feet and, like many modern structures, it is made of concrete reinforced with steel rebar. This combination is popular because it is strong, inexpensive and easy to construct, and, most importantly, because steel and concrete have very similar coefficients of linear expansion, about 1.1×10−5 ( ◦C)−1 . The llocations highest recorded temperature is 38.3◦C. Its lowest recorded temperature is -23.3◦C 1 (a) How much will the length of the bridge change between the highest and lowest temperatures? (b) The bridge is not a single piece of concrete, but rather is made of many slabs supported by a steel structure. Why do you think that is? (c) If steel had a very different coefficient of thermal expansion compared to concrete, what do you think would happen to steel-reinforced concrete over the course of a year?arrow_forwardA bicycle tire has a pressure of 7.00 X 105 N/m2 at a temperature of 18.0oC and contains 2.00 L of gas. What will its pressure be if you let out an amount of air that has a volume of 100 cm3 at atmospheric pressure? Assume tire temperature and volume remain constant.arrow_forwardA brass rod of length 50 cm and diameter 3.0 mm is joined to a steel rod of the same length and diameter. What is the change in length of the combined rod at 250 °C, if the original lengths are at 40.0 °C? Is there a ‘thermal stress’ developed at the junction ? The ends of the rod are free to expand (Co-efficient of linear expansion of brass = 2.0×10-5 K-1 steel =1.2×10-5 K-1arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning


