
(a)
Interpretation:
The reactivity of cyclohexanol and phenol with aqueous
Concept introduction:
The phenol is an aromatic compound having a formula,
Answer to Problem 18.53AP
Phenol is more reactive than cyclohexanol with an aqueous
Explanation of Solution
The sodium hydroxide is a strong base. It abstracts a proton from the stronger acidic compound and forms its sodium salt. In the given case, sodium hydroxide abstracts a proton from phenol and cyclohexanol and form phenoxide ion and cyclohexanol conjugate base respectively. The phenoxide ion gets stabilized by resonance structures, but this is not possible in case of cyclohexanol conjugate base. It is known that more the stability of the conjugate base more the acidity of the compound. In the given case, phenoxide ion is more stable due to this phenoxide is more acidic. Therefore, phenoxide ion is more reactive toward an aqueous
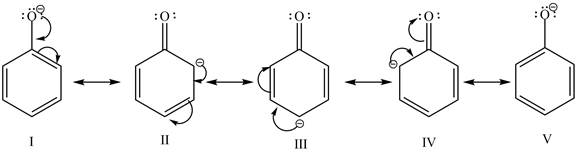
Figure 1
The reactivity of phenol is more as compared to cyclohexanol with an aqueous solution of
(b)
Interpretation:
The reactivity of cyclohexanol and phenol with
Concept introduction:
The phenol is an aromatic compound having a formula,
Answer to Problem 18.53AP
Phenol is more reactive than cyclohexanol with an aqueous
Explanation of Solution
The sodium hydride is a strong base. It abstracts a proton from the stronger acidic compound and forms its sodium salt and hydrogen gas is evolved. In the given case, sodium hydroxide abstracts a proton from phenol and cyclohexanol and form phenoxide ion and cyclohexanol conjugate base respectively. The phenoxide ion gets stabilized by resonance structures, but this is not possible in case of cyclohexanol conjugate base. It is known that more the stability of the conjugate base more the acidity of the compound. In the given case, phenoxide ion is more stable due to this phenoxide is more acidic. Therefore, phenoxide ion is more reactive toward an aqueous
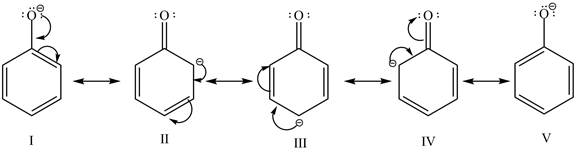
Figure 2
The reactivity of phenol is more as compared to cyclohexanol with an aqueous solution of
(c)
Interpretation:
The reactivity of cyclohexanol and phenol with triflic anhydride in pyridine at
Concept introduction:
The triflic anhydride is a chemical compound which is also known as Trifluoromethanesulfonic anhydride. It has a molecular formula
Answer to Problem 18.53AP
The presence of the more nucleophilic character of cyclohexanol makes it more reactive towards triflic anhydride in pyridine at
Explanation of Solution
In the given case, when triflic acid reacts with cyclohexanol and phenol in the presence of pyridine at
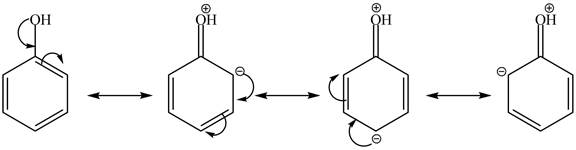
Figure 3
The reactivity of cyclohexanol is more as compared to phenol with triflic acid in pyridine at
(d)
Interpretation:
The reactivity of cyclohexanol and phenol with concentrated aqueous
Concept introduction:
The phenol is an aromatic compound having a formula,
Answer to Problem 18.53AP
The deactivation of the aromatic ring of phenol makes it less reactive toward concentrated aqueous
Explanation of Solution
Cyclohexanol is more nucleophilic as compared to phenol. It is due to the participation of the lone pair of electrons on oxygen in resonance structures of phenol. Therefore, in the given conditions cyclohexanol reacts more rapidly as compared to phenol. When cyclohexanol undergoes protonation in the presence of
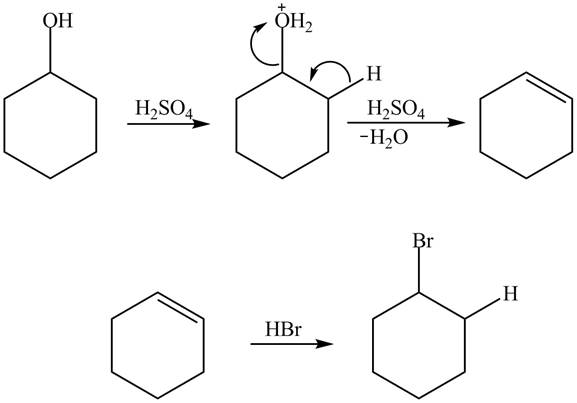
Figure 4
In the case of phenol, it undergoes electrophilic aromatic substitution reaction with
The reactivity of phenol is less with concentrated aqueous
(e)
Interpretation:
The reactivity of cyclohexanol and phenol with
Concept introduction:
The phenol is an aromatic compound having a formula,
Answer to Problem 18.53AP
The phenol is more reactive toward
Explanation of Solution
The phenol is an aromatic compound which shows similar reactions as
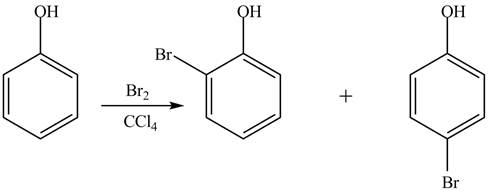
Figure 5
Whereas no such reaction is possible with cyclohexanol because this compound gives addition reaction.
The phenol is an aromatic compound which undergoes electrophilic substitution reaction. Due to this, it is more reactive toward
(f)
Interpretation:
The reactivity of cyclohexanol and phenol with
Concept introduction:
Oxidation is defined as the addition of oxygen atom or removal of the hydrogen atom. The oxidizing agent is the substance that causes oxidation and itself get reduced. The reagent
Answer to Problem 18.53AP
The phenol is an aromatic compound which loses its aromatic character on reaction with
Explanation of Solution
In the given conditions, both the given compounds undergo an oxidation reaction. The reaction of cyclohexanol with
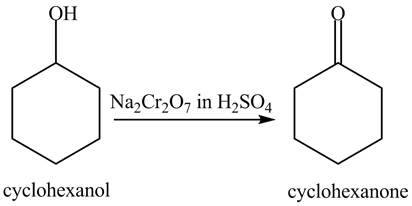
Figure 6
The reaction of phenol with
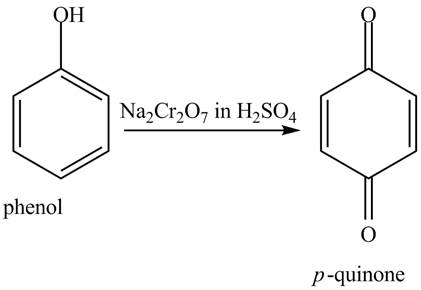
Figure 7
When phenol undergoes oxidation reaction, it loses its aromatic character which means it loses its stability. Therefore, cyclohexanol is more reactive toward
The phenol on reaction with
(g)
Interpretation:
The reactivity of cyclohexanol and phenol with
Concept introduction:
The phenol is an aromatic compound having a formula,
Answer to Problem 18.53AP
The deactivation of the aromatic ring of phenol due to protonation of the hydroxyl group makes it less reactive toward
Explanation of Solution
Cyclohexanol is more nucleophilic as compared to phenol. It is due to the participation of the lone pair of electrons on oxygen in resonance structures of phenol. Therefore, in the given conditions cyclohexanol reacts more rapidly as compared to phenol. When cyclohexanol undergoes protonation in the presence of
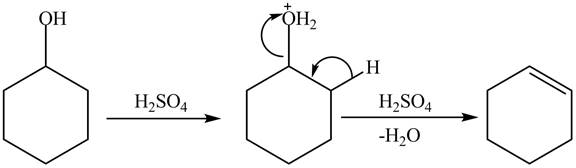
Figure 8
The less nucleophilic character makes phenol less reactive toward
The reactivity of phenol is less with
Want to see more full solutions like this?
Chapter 18 Solutions
Loose-leaf Version For Organic Chemistry
- Arrange the following molecules in increasing order of acidity. Base it only on their structural differences and explain how it is so. 1. HF, CH3CH2CH2OH, CH3CH2COOH 2. Ethyl amine, Ethanol, Propanearrow_forwardWhen the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forwardAssume that you have samples of the following two compounds, both with formula C7H8O. Both compounds dissolve in ether, but only one of the two dissolves in aqueous NaOH. How could you use this information to distinguish between them?arrow_forward
- Draw the structures for and give the names for each of the isomeric carbonyl compounds corresponding to C4H8O and suggest suitable reactions for distinguishing between themarrow_forwardA synthetic organic molecule, G, which contains both aldehyde and ether functional groups, is subjected to a series of reactions in a multi-step synthesis pathway. In the first step, G undergoes a Wittig reaction, leading to the formation of an alkene, H. Subsequently, H is treated with an ozone (O3) reagent followed by a reducing agent in an ozonolysis reaction, resulting in the formation of two different products, I and J. Considering the functional groups present in G and the nature of the reactions involved, what are the most probable structures or functional groups present in products I and J? A. I contains a carboxylic acid group, and J contains an aldehyde group. B. I contains a ketone group, and J contains an alcohol group. C. I and J both contain aldehyde groups. D. I contains an ester group, and J contains a ketone group. Don't use chat gpt.arrow_forwardIsoerythrogenic acid, C18H26O2, is a acetylic fatty acid that turns vivid vle on exposure to UV light. On Catalytic hydrogenation over a palladium catalyst, five molar equivalents of hydrogen are absorbed, and stearic acidarrow_forward
- If N,N-diethyl-1-pentanamine reacts with 3-Methylhexanoic acid, draw the resulting skeletal structure and provide its name?arrow_forwardPredict the products and proposemechanisms for the reactions ofcarboxylic acids with reducingagents, alcohols, amines, andorganometallic reagentsarrow_forwardWrite the chemical equation for the acid dissociation of acetaminophen, C8H9O2N. Write the Ka expression for the acid dissociation of acetaminophen.arrow_forward
- What are 2-3 organic compounds that each contain at least one of the following: carboxylic acid, ester, amine, or amide. How it is synthesized and used, and how the function of the compound is facilitated by its structure and, specifically, its functional groups.arrow_forwardIn an advanced synthetic chemistry experiment, a researcher prepares a compound, ZY-7, by reacting a ketone (C5H10O) with hydroxylamine (NH2OH), followed by heating in the presence of an acid catalyst. The resulting compound, ZY-7, is then treated with a solution of sodium nitrite (NaNO2) and hydrochloric acid (HCl) at low temperature. Identify the class of compound that ZY-7 most likely belongs to after this series of reactions." A) Amide B) Oxime C) Nitro compound D) Diazonium salt E) Ester Don't use chatgpt please provide valuable answerarrow_forwardAcid-catalyzed hydration of 2-Methyl-1-butene yields two alcohols. The major product does not undergo oxidation, while the minor product will undergo oxidation. Explain why, by showing the structures of theproducts.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



