
Concept explainers
Synthesize each compound from benzene and any other organic or inorganic reagents.
a. 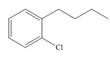 c.
c. 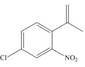 e.
e. 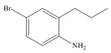 g.
g. 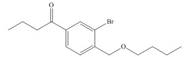
b. 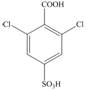 d.
d. 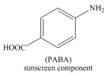 f.
f. 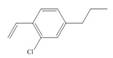 h.
h. 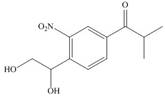
(a)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.63P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
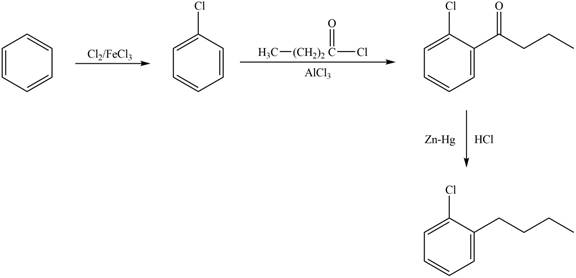
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 1.
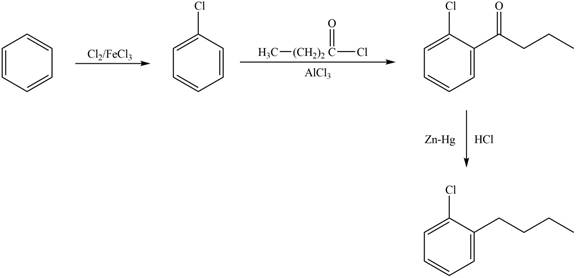
Figure 1
The synthesis of given compound takes place by chlorination of benzene, Friedel-Craft acylation and clemmensen reduction at last step.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 1.
(b)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.63P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
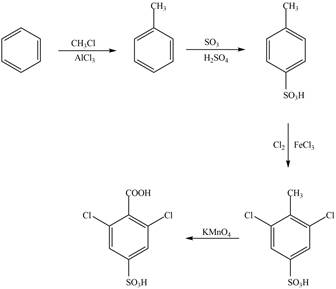
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 2.
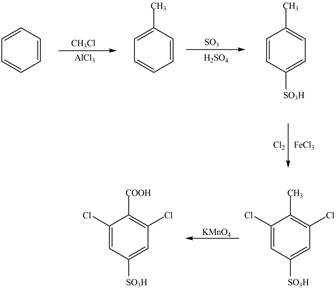
Figure 2
The synthesis of given compound take place in four steps: Friedel-Craft alkylation, sulfonation, chlorination and at last oxidation.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 2.
(c)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.63P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
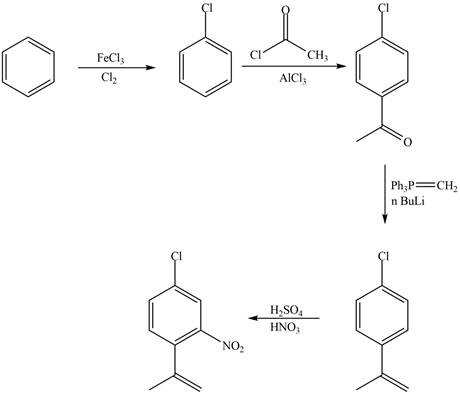
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 3.
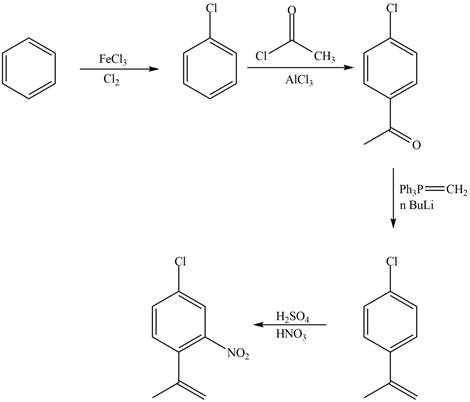
Figure 3
The first, second, third and fourth step involved in the synthesis of given compound is chlorination, Friedel-Craft acylation, Wittig reaction and nitration, respectively.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 3.
(d)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.63P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
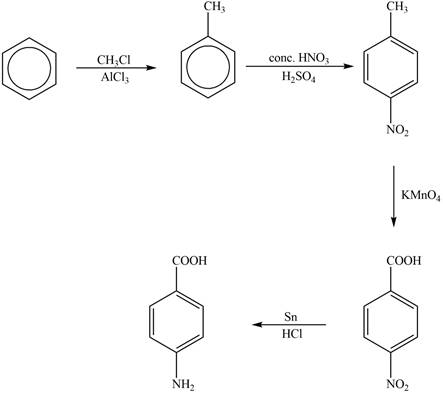
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 4.
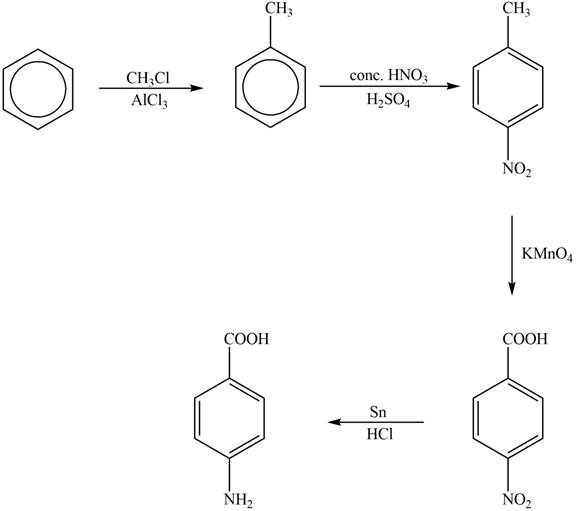
Figure 4
The first step of the synthesis is Friedel-Craft alkylation reaction. The product of this reaction undergoes nitration reaction. In next step, the
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 4.
(e)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.63P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
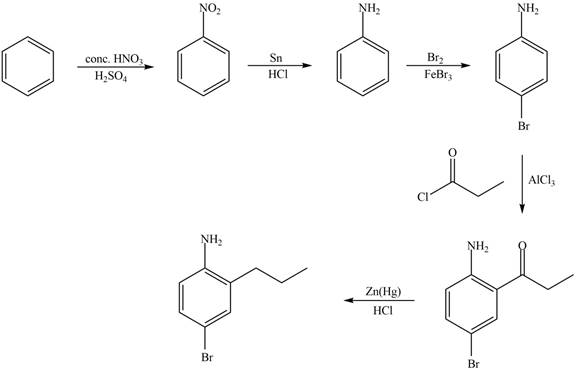
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 5.
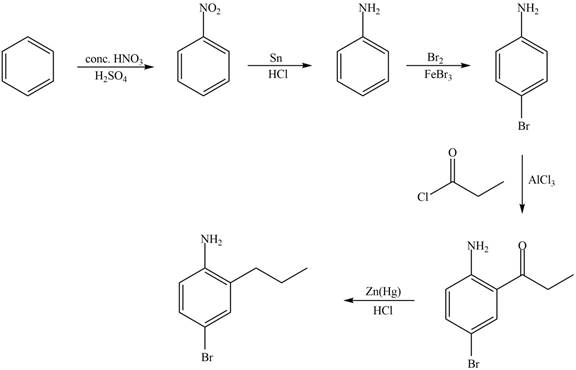
Figure 5
Benzene undergoes nitration on reaction with
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 5.
(f)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.63P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
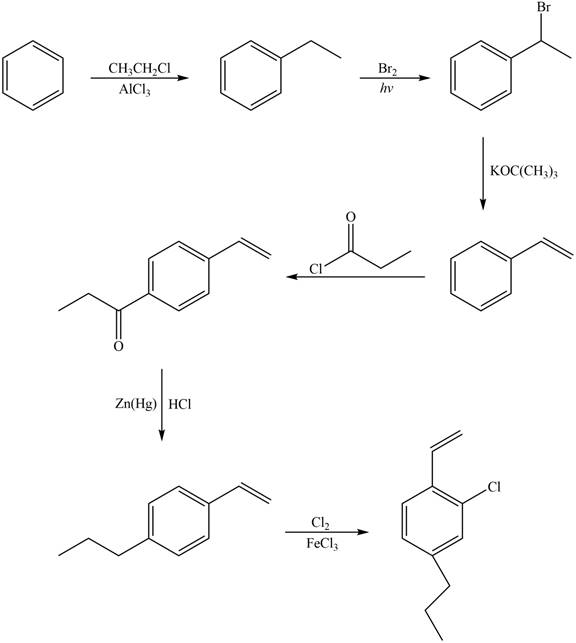
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 6.
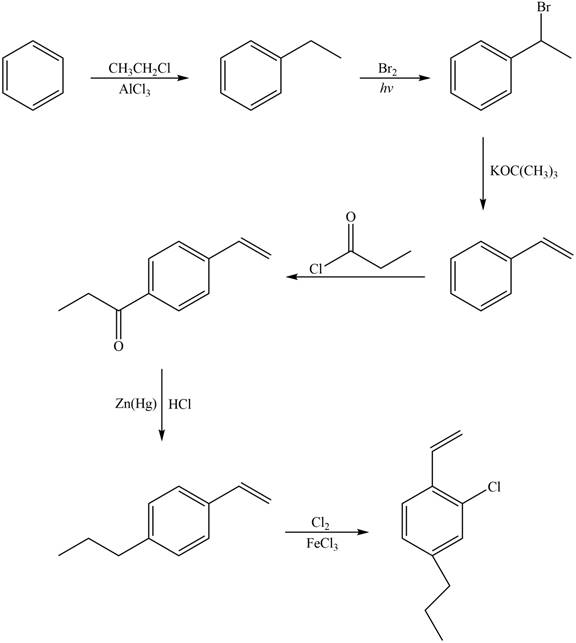
Figure 6
Benzene on Friedel-Craft alkylation with ethylchloride gives ethylbenzene. This product undergoes bromination in presence of light. The next step involves the abstraction of bromine on reaction with tertiary butoxide. This leads to the formation of
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 6.
(g)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.63P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
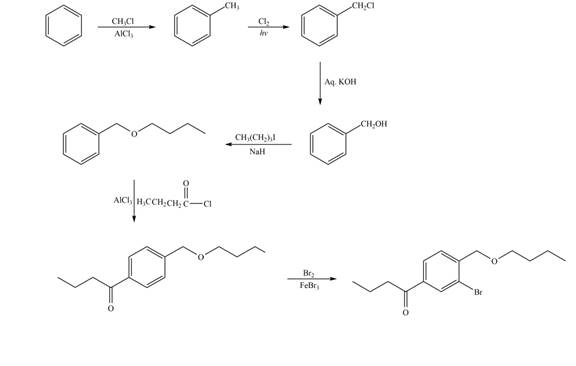
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 7.
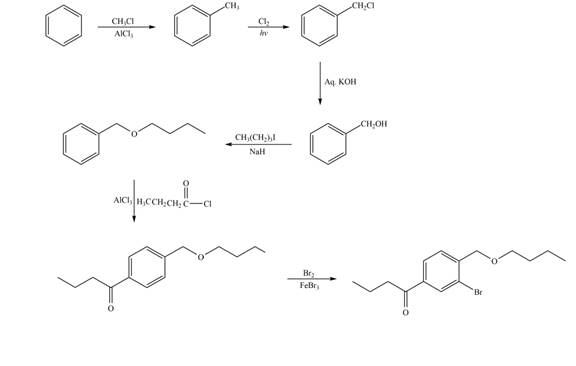
Figure 7
The first two steps involved in the synthesis of given compound are Friedel-Craft alkylation followed by chlorination in the presence of light. The chlorine group is replaced by hydroxyl group on reaction with
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 7.
(h)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.63P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
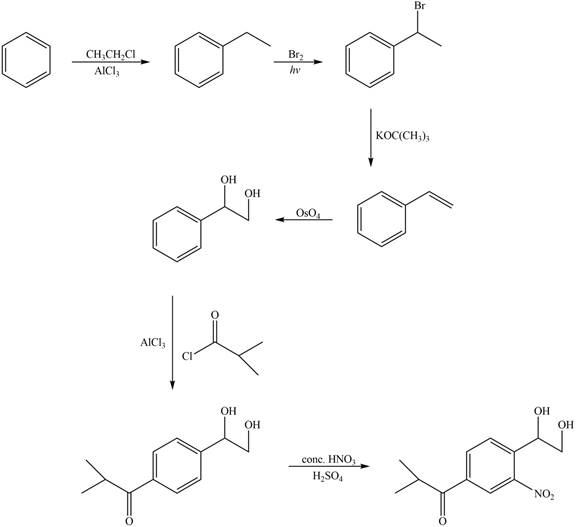
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 8.

Figure 8
Benzene on Friedel-Craft alkylation with ethylchloride gives ethylbenzene. This product undergoes bromination in presence of light. The next step involves the abstraction of bromine on reaction with tertiary butoxide. This leads to the formation of
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 8.
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry
- Devise a synthesis of each compound from cyclohexene and any required organic or inorganic reagents.arrow_forwardDevise a synthesis of each compound from cyclopentane and any other required organic or inorganic reagents.arrow_forwardDevise a synthesis of each alkyne. You may use acetylene, benzene, organic halides, ethylene oxide, and any other required inorganic reagents.arrow_forward
- Devise a synthesis of attached compound from acetylene and any other required reagents.arrow_forwardDevise a synthesis of attached alkyne. You may use acetylene, benzene,organic halides, ethylene oxide, and any other required inorganicreagents.arrow_forwardDevise a synthesis of each compound from phenol (C6H5OH) and any other organic or inorganic reagents.arrow_forward
- Devise a synthesis of attached compound from benzene, any organic alcohols having four or fewer carbons, and any required reagentsarrow_forwardDraw a stepwise mechanism for the attached reaction that forms ether D. D can be converted to the antidepressant fluoxetine (trade name Prozac) in a single steparrow_forwardDevise a synthesis of each compound from benzene, any organic alcohols having four or fewer carbons, and any required reagents.arrow_forward
- Devise a synthesis of each compound from benzene and organicalcohols containing four or fewer carbons. You may also use anyrequired organic or inorganic reagents.arrow_forwardstructures for compounds A–F.arrow_forwardDevise a synthesis of attached biologically active compound from benzenearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





