
Interpretation:
The short sequences of reactions that would be appropriate for the given transformations, using the indicated starting material are to be written.
Concept introduction:
Friedel Craft acylation is an electrophilic
Grignard reaction is a reaction in which aryl or
The molecule,
Dess Martin periodinane (DMP) is a reagent which is used for the oxidation of primary alcohols to
The Diels–Alder reaction is a
Answer to Problem 42P
Solution:
a) Short sequences of reactions that are appropriate for the transformation of
b) Short sequences of reactions for the transformation of the given compounds are shown below.
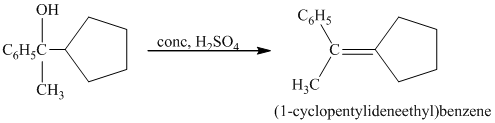
c) Short sequences of reactions for the transformation of the given compound from
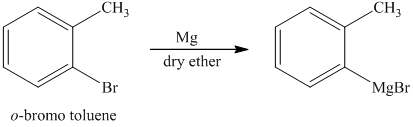
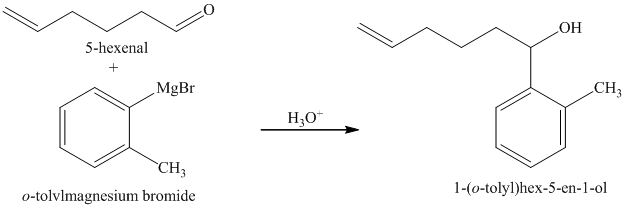
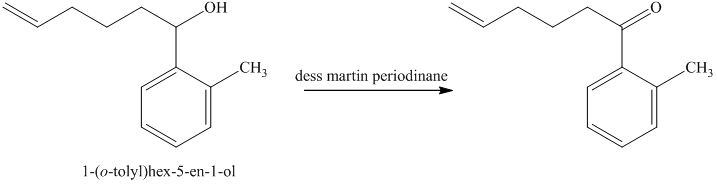
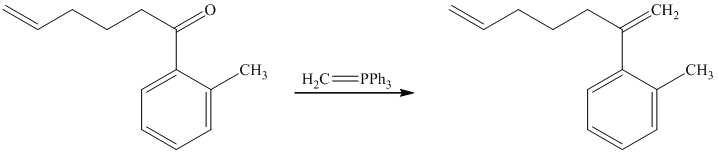
d) Short sequences of reactions for the transformation of the given compounds are shown below.

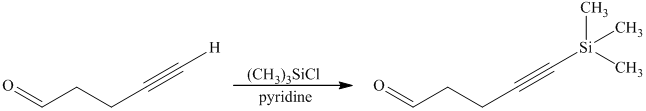
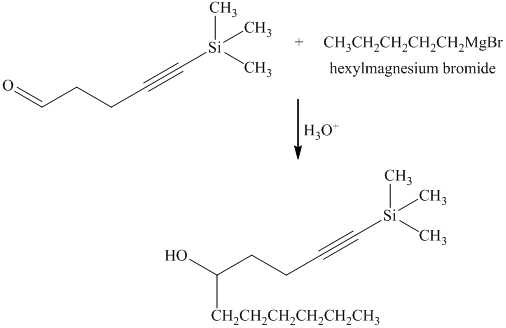
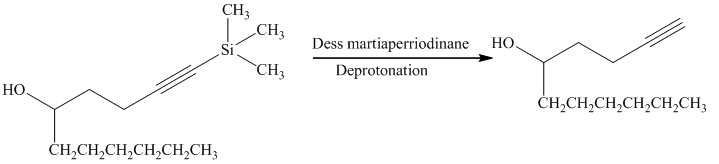
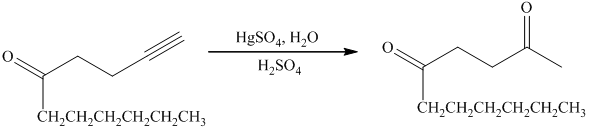
e) Short sequences of reactions that are appropriate for the transformation of the given compound from
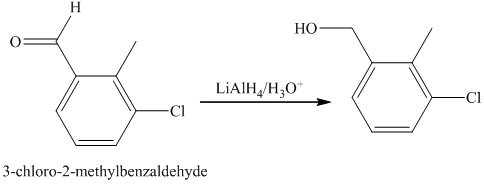
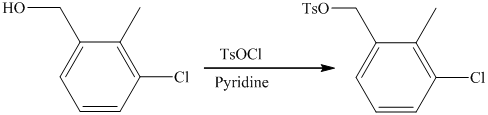
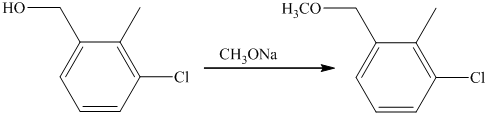
f) Short sequences of reactions that are appropriate for the transformation of the given compounds are shown below.
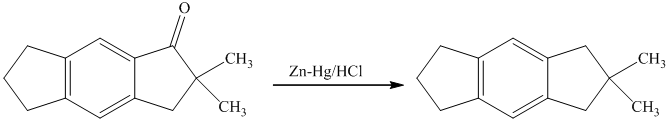
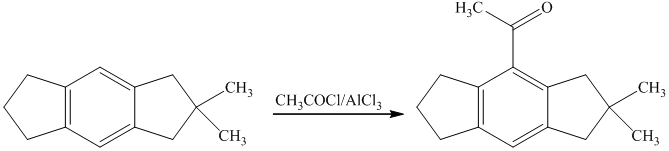
g) Short sequences of reactions that are appropriate for the transformation of the given compounds are shown below.
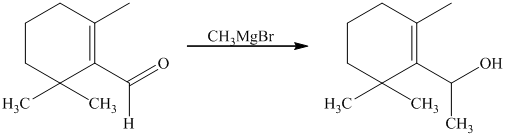
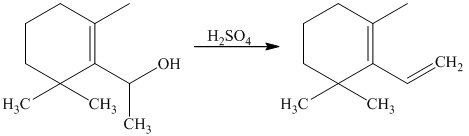
Explanation of Solution
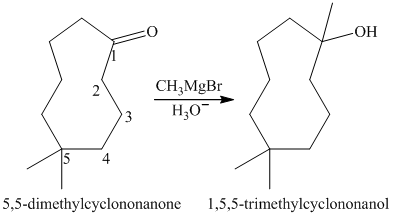
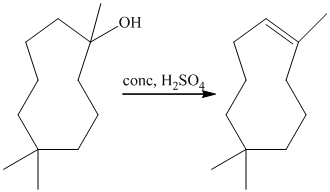
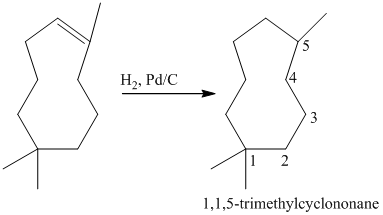
a) Short sequences of reactions that would be appropriate for the transformation of
Treatment of
The tertiary alcohol so obtained undergoes dehydration with conc.

Reduction of the alkene with

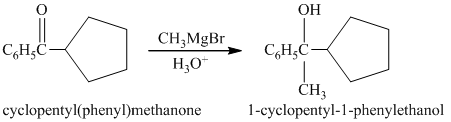
b) Short sequences of reactions that would be appropriate for the transformation of the compounds given below have to be decribed.
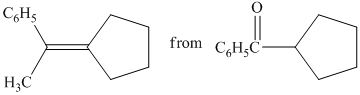
Treatment of cyclopentyl(phenyl) methanone with griginard reagent

The tertiary alcohol, upon dehydration with conc.

c) The structure of the compound which has to be synthesized from
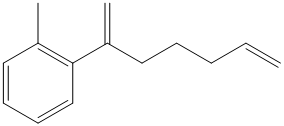
The reaction of

The griginard reagent formed reacts with

The alcohol formed reacts with dess martin periodinane to form ketone as shown in the reaction below.

The ketone reacts with Wittig reagent

d) Short sequences of reactions that would be appropriate for the transformation of the compounds given below have to be described.
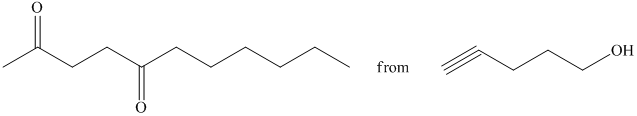
Oxidation of the given alcohol using pyridinium dichromate (PDC) in

Protection of acidic proton of is done by reaction of the aldehyde with

Reaction of the aldehyde with the griginard reagent, hexylmagnesium chloride, produces an alcohol as shown in the reaction below.

Alcohols react with martin dess periodinane to form ketone as shown below.

Terminal alkynes are converted to ketones by reaction with

e) The structure of the compound which has to be synthesized from
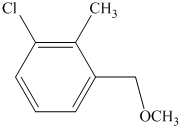
In the reduction of

The hydroxyl group of the alcohol is a poor leaving group and therefore, it is reacted with

The

f) Short sequences of reactions that would be appropriate for the transformation of the compounds given below have to be decribed.
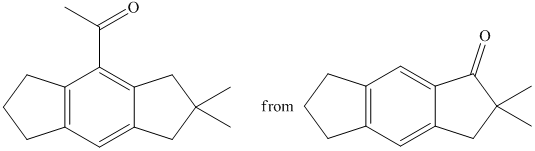
Reduction of the ketone shown above with zinc-amalgam produces a hydrocarbon in Clemmenson’s reduction as shown in the reaction below.

Friedal Crafts acylation of the aromatic ring obtained with

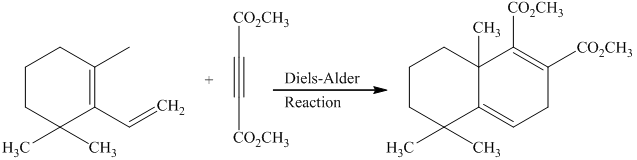
g) Short sequences of reactions that would be appropriate for the transformation of the compounds given below have to be decribed.
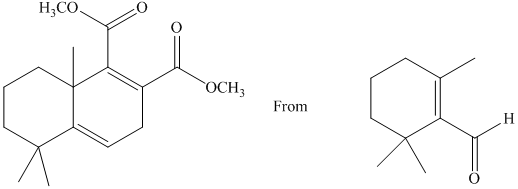
Treatment of the reactant with griginard reagent

The tertiary alcohol so obtained undergoes dehydration with conc.

Diels-Alder reaction of the diene with the dienophile produces the desired product as shown in the reaction below.

Want to see more full solutions like this?
Chapter 18 Solutions
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
- Following is a retrosynthetic analysis for an intermediate in the industrial synthesis of vitamin A. (a) Addition of one mole of HCl to isoprene gives 4-chloro-2-methyl-2-butene as the major product. Propose a mechanism for this addition and account for its regioselectivity. (b) Propose a synthesis of the vitamin A precursor from this allylic chloride and ethyl acetoacetate.arrow_forwardThree isomeric pentanols with unbranched carbon chains exist. Which of these isomers, upon dehydration at 180C, yields only 1-pentene as a product?arrow_forwardThe base-promoted rearrangement of an -haloketone to a carboxylic acid, known as the Favorskii rearrangement, is illustrated by the conversion of 2-chlorocyclohexanone to cyclopentanecarboxylic acid. It is proposed that NaOH first converts the a-haloketone to the substituted cyclopropanone shown in brackets and then to the sodium salt of cyclopentanecarboxylic acid. (a) Propose a mechanism for base-promoted conversion of 2-chlorocyclohexanone to the proposed intermediate. (b) Propose a mechanism for base-promoted conversion of the proposed intermediate to sodium cyclopentanecarboxylate.arrow_forward
- The following sequence of steps converts (R)-2-octanol to (S)-2-octanol. Propose structural formulas for intermediates A and B, specify the configuration of each, and account for the inversion of configuration in this sequence.arrow_forwardthe organic compound 2-heptanone, belonging to the ketone family, is responsible for the strong penetrating odor in Roquefort cheeses. Starting from acetylene as the starting reagent, propose a synthesis line with the reaction mechanisms involved for the synthetic obtaining of 2-heptanone and use it as a food additive in analogous cheeses.arrow_forwardWhen 5-bromo-1-pentanol is treated with sodium hydride in diethyl ether, the product is analyzed to be C5H10O. Propose a likely structure for this product, suggesting a reasonable mechanistic pathway for its formationarrow_forward
- This problem is adapted from an experiment designed for undergraduate organic chemistry laboratories. (a) Reaction of (E)-1-(p-methoxyphenyl)propene with m-chloroperoxybenzoic acid converted the alkene to its corresponding epoxide. Give the structure, including stereochemistry, of this epoxide.(b) Assign the signals in the 1H NMR spectrum of the epoxide to the appropriate hydrogens. δ 1.4 (doublet, 3H) δ 3.8 (singlet, 3H) δ 3.0 (quartet of doublets, 1H) δ 6.9 (doublet, 2H) δ 3.5 (doublet, 1H) δ 7.2 (doublet, 2H) (c) Three signals appear in the range δ 55–60 in the 13C NMR spectrum of the epoxide. To which carbons of the epoxide do these signals correspond? (d) The epoxide is isolated only when the reaction is carried out under conditions (added Na2CO3) that ensure that the reaction mixture does not become acidic. Unless this precaution is taken, the isolated product has the molecular formula C17H17O4Cl. Suggest a reasonable structure for this product and write a reasonable mechanism…arrow_forward1. Compound A, C9H12, absorbs 3 equivalents of H2 on catalytic hydrogenation over palladium catalyst to give B, C9H18. On the treatment with acidic KMnO4, compound A gives among other things, a ketone that was identified as cyclohexanone. On reaction with NaNH2 in NH3, followed by addition of iodomethane, compound A gives a new hydrocarbon C, C10H14. What are the structures of A , B and C?arrow_forwardThe p-toluenesulfonate derived from (R)-2-octanol and p-toluenesulfonyl chloride was allowed to react with sodium benzenethiolate (C6H5SNa). Give the structure, including stereochemistry and the appropriate R or S descriptor, of the product.arrow_forward
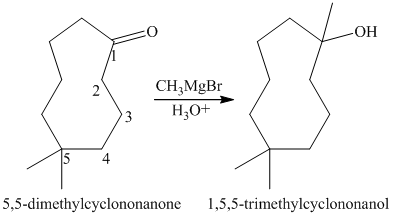
- Mention and Write the following reaction mechanisms: a) thermal dehydration of 4-t-butylcyclohexanol with phosphoric acid as catalyst; b) addition of molecular bromine to the C= C double bond of 4-t- butylcyclohexene;arrow_forwardSyntheses of each of the following compounds have been reported in the chemical literature. Using the indicated starting material and any necessary organic or inorganic reagents, describe short sequences of reactions that would be appropriate for each transformation. (a) 1,1,5-Trimethylcyclononane from 5,5-dimethylcyclononanonearrow_forwardA solution of acetone [(CH3)2C=O] in ethanol (CH3CH2OH) in the presence of a trace of acid was allowed to stand for several days, and a new compound of molecular formula C7H16O2 was formed. The IR spectrum showed only one major peak in the functional group region around 3000 cm−1, and the 1H NMR spectrum is given here. What is the structure of the product?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,



