
(a)
Pressure and volume at given points.
(a)
Answer to Problem 61P
Volume at A, B, C, D are
Pressure at B and D are
Explanation of Solution
Write the ideal gas equation and rearrange for
Here
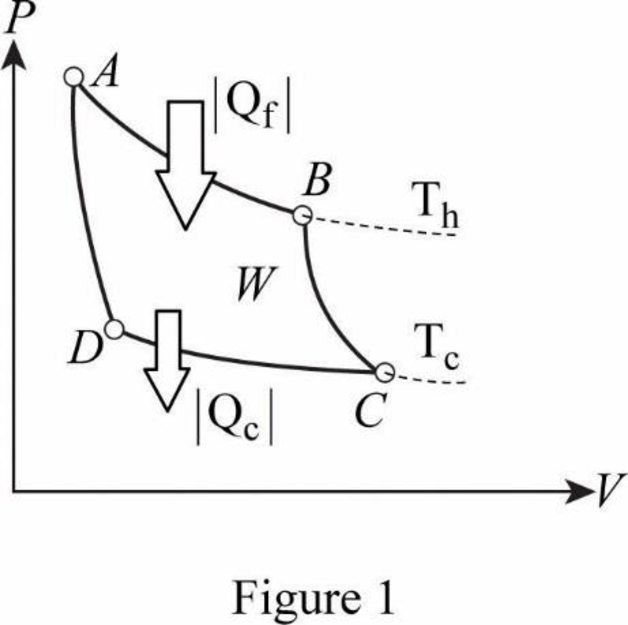
From figure, AB is isothermal.
Therefore.
Here
BC is adiabatic.
Therefore,
Here
Rearrange (II) in terms of
Rearrange (IV) in terms of
Similarly,
CD is isothermal.
Here
AD is adiabatic.
Substitute for
Rearrange (VIII) in terms of
Rearrange (II) and (VI) in terms of
Conclusion:
Substitute
Substitute
Substitute
Substitute
Volume at A, B, C, D are
Pressure at B and D are
(b)
Net work done per cycle.
(b)
Answer to Problem 61P
Net work done is
Explanation of Solution
Write the first law of
Here
As the change in internal energy is zero, (XI) becomes,
Write the equation for work done
Here
Write the equation for efficiency
Here
Rewrite (XIIII) in terms of
Conclusion:
Substitute
Net work done is
Want to see more full solutions like this?
Chapter 18 Solutions
Principles of Physics
- Does the area enclosed by the cycle on a T-s diagram represent the net work input for the reversed Carnot cycle? How about for the ideal vapor-compression refrigeration cycle?arrow_forwardA sample containing 1.00 kmol of helium (treated as an ideal gas) is putthrough the cycle of operations shown in the figure. BC is an isotherm,and pA = 1.00 atm, VA = 22.4 m3, pB = 2.00 atm. a) Calculate the temperatures TA, TB and volume VC. b) Calculate the work done during the cycle. Recall the expression for work done during an isothermalprocess.arrow_forwardConsider a monatomic ideal gas operating through the Carnot cycle. The initial volume of the gas is V1 = 130 × 10-3 m3. What types of processes are going on for each step in this process? During the isothermal compression step, the volume of gas is reduced by a factor of 4. In the adiabatic heating step, the temperature of the gas is doubled. What is the volume at point 3, in cubic meters?arrow_forward
- Work is entropy free, and sometimes the claim is made that work will not change the entropy of a fluid passing through an adiabatic steady-flow system with a single inlet and outlet. Is this a valid claim?arrow_forwardAn engineer decided to design a hybrid engine half-way between the Carnot and Otto Cycles based on using 35 moles of a diatomic gas. It features (a) an adiabatic expansion for power, (b) an isothermal compression to reset, and (c) an isovolumetric temperature injection. Some of the key state values are given on the P-V chart for the transition points between the processes. What is the 1) net work performed by the engine, 2) the amount of heat injected the isovolumetric temperature increase (QH), and 3) based on these values, what is the efficiency of the engine?Solve for other state variables as necessary How much more efficient would a Carnot engine be acting between these same temperatures?arrow_forwardRecall Problem 1.34, which concerned an ideal diatomic gas taken around a rectangular cycle on a PV diagram. Suppose now that this system is used as a heat engine, to convert the heat added into mechanical work. (a) Evaluate the efficiency of this engine for the case V2 = 3V1 , P2 = 2P1. (b) Calculate the efficiency of an "ideal" engine operating between the same temperature extremes.arrow_forward
- A closed thermodynamic cycle described by an ellipse is shown on the PV diagram on the right. The maximum pressure of the system is 8.0 x 105 Pa, and the minimum pressure of the system is 4.0 x 10^5 Pa. The maximum volume of the system is 4.0 x 10^-3 m^3 and the minimum volume of the system is 2.0 x 10^-3 m^3 . a) Calculate the magnitude of the total work done by the system in one full cycle. b) Does this thermodynamic cycle describe what happens in an ENGINE or a REFRIDGERATOR/HEAT-PUMP?arrow_forwardA perfect gas at 727 oC and with a volume of 0.003 m3expands reversibly in a cylinder behind a piston until its temperature is 2 oC and volume is 0.6 m3according to a linear relationship on the T-s diagram such that its entropy increases as the temperature decreases. Sketch the process on the T-s diagram and calculate the work transfer per kilogram of gas. The specific heat capacity at constant volume, cv is 1.23 kJ/kg K and the specific gas constant, R is 0.3198 kJ/kg Karrow_forwardConsider a two-step process where 2.00 mol argon is (1) heated at constant pressure from 300. K to 400. K, then (2) compressed isothermally until its volume is halved. Calculate the change in entropy for step 1 and 2 individually first, then the total entropy change. Comment on what is happening at each step and overall. Assume Cp = 5/2 R for a monatomic ideal gas. (answers: step 1 = 11.96 J/K; step 2 = -11.53 J/K; total = 0.43 J/K)arrow_forward
- 2.0 mol of a monatomic ideal gas are at a temperature of 300 K and a pressure of 1.0 atm. What is the entropy change in a process that brings the gas to 350 K and 1.3 atm? Express your answer with the appropriate units.arrow_forwardThree Carnot engines operate between temperature limits of (a) 400 and 500 K, (b) 500 and 600 K, and (c) 400 and 600 K. Each engine extracts the same amount of energy per cycle from the high-temperature reservoir. Rank the magnitudes of the work done by the engines per cycle, greatest first.arrow_forwardA heat engine takes 3 moles of an ideal gas through the reversible cycle abca, on the pV diagram shown in the figure. The path bc is an isothermal process. The temperature at c is 700. K, and the volumes at a and c are 0.020 m3 and 0.25 m3, respectively. The molar heat capacity at constant volume, of the gas, is 20 J/mol·K, and the ideal gas constant is R = 8.314 J/(mol∙K). What is the thermal efficiency of this engine?arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning



