
Physics For Scientists and Engineers
4th Edition
ISBN: 9780134391786
Author: Randall D. Knight
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 19, Problem 20EAP
An experiment measures the temperature of a 500 g substance while steadily supplying heat to it. FIGURE EX19.20 shows the results of the experiment. What are the (a) specific heat of the solid phase, (b) specific heat of the liquid phase, (c) melting and boiling temperatures, and (d) heats of fusion and vaporization?
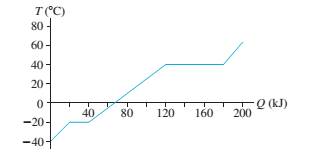
FIGURE EX19.20
.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The heat of evaporation of water at atmospheric pressure is Lv 2260 kJ/kg.
a. How much of this heat represents work done to expand the water into steam against the pressure of the atmosphere?
b. What becomes of the rest of the heat? At T= 100°C and p=1.0 atm, the density of water is 1.00 × 10³ kg/m³ and the density of steam is 0.600kg/m³?
How much heat is removed from the body when 300-g ice from an ice pack melted and the water inside had a temperature of 25°C?
a. 3135 J
b. 31 350 J
c. 100 080 J
d. 131 430 J
In what conditions will vaporization best occur?
a. low kinetic energy, strong molecular forces, and large surface area
b. high kinetic energy, weak molecular forces, and large surface area
c. low kinetic energy, low molar mass, and small surface area
d. high kinetic energy, high molar mass, and large surface area
Assuming all substances are at room temperature, choose the liquid having a vapor pressure between higher
a. mercury and water
b. condensed milk and evaporated milk
c. acetone and honey
d. motor oil and gasolinens
To illustrate the effect of ice on the aluminum cooling plate, consider the drawing shown here and the data that it contains. Ignore any limitaions due to significant figures. How much heat per second per square meter is conducted through the ice-aluminum combination and through the aluminum by itself?
combination: 6580 J/(s m2), aluminum only: 6580 J/(s m2)
combination: 2400000 J/(s m2), aluminum only: 2400000 J/(s m2)
combination: 2400000 J/(s m2), aluminum only: 6580 J/(s m2)
combination: 6580 J/(s m2), aluminum only: 2400000 J/(s m2)
Chapter 19 Solutions
Physics For Scientists and Engineers
Ch. 19 - Prob. 1CQCh. 19 - Do (a) temperature, (b) heat, and (c) thermal...Ch. 19 - Prob. 3CQCh. 19 - You need to raise the temperature of a gas by...Ch. 19 - Prob. 5CQCh. 19 - Prob. 6CQCh. 19 - FIGURE Q19.7 shows two different processes taking...Ch. 19 - FIGURE Q19.8 shows two different processes taking...Ch. 19 - The gas cylinder in FIGURE Q19.9 is a rigid...Ch. 19 - The gas cylinder in FIGURE Q19.10 is well...
Ch. 19 - The gas cylinder in FIGURE Q19.11 is well...Ch. 19 - How much work is done on the gas in the process...Ch. 19 - Prob. 2EAPCh. 19 - Prob. 3EAPCh. 19 - A 2000 cm3 container holds 0.10 mol of helium gas...Ch. 19 - Prob. 5EAPCh. 19 - Prob. 6EAPCh. 19 - Draw a first-law bar chart (see Figure 19.12) for...Ch. 19 - Draw a first-law bar chart (see Figure 19.12) for...Ch. 19 - 9. Draw a first-law bar chart (see Figure 19.12)...Ch. 19 - Prob. 10EAPCh. 19 - J of work are done on a system in a process that...Ch. 19 - How much heat energy must be added to a...Ch. 19 - Prob. 13EAPCh. 19 - Prob. 14EAPCh. 19 - Prob. 15EAPCh. 19 - Prob. 16EAPCh. 19 - One way you keep from overheating is by...Ch. 19 - Prob. 18EAPCh. 19 - Two cars collide head-on while each is traveling...Ch. 19 - An experiment measures the temperature of a 500 g...Ch. 19 - 30 g of copper pellets are removed from a 300°C...Ch. 19 - A 750 g aluminum pan is removed from the stove and...Ch. 19 - A 50.0 g thermometer is used to measure the...Ch. 19 - A 500 g metal sphere is heated to 300°C, then...Ch. 19 - A 65 cm3 block of iron is removed from an 800°C...Ch. 19 - Prob. 26EAPCh. 19 - A container holds 1.0 g of oxygen at a pressure of...Ch. 19 - The volume of a gas is halved during an adiabatic...Ch. 19 - Prob. 29EAPCh. 19 - Prob. 30EAPCh. 19 - Prob. 31EAPCh. 19 - Prob. 32EAPCh. 19 - Prob. 33EAPCh. 19 - Prob. 34EAPCh. 19 - Prob. 35EAPCh. 19 - What maximum power can be radiated by a...Ch. 19 - Radiation from the head is a major source of heat...Ch. 19 - Prob. 38EAPCh. 19 - Prob. 39EAPCh. 19 - Prob. 40EAPCh. 19 - Prob. 41EAPCh. 19 - Prob. 42EAPCh. 19 - Prob. 43EAPCh. 19 - The specific heat of most solids is nearly...Ch. 19 - Prob. 45EAPCh. 19 - Prob. 46EAPCh. 19 - Prob. 47EAPCh. 19 - Prob. 48EAPCh. 19 - .0 mol of gas are at 30°C and a pressure of 1.5...Ch. 19 - A 6.0-cm-diameter cylinder of nitrogen gas has a...Ch. 19 - Prob. 51EAPCh. 19 - An ideal-gas process is described by p = cV 1/2 ,...Ch. 19 - Prob. 53EAPCh. 19 - Prob. 54EAPCh. 19 - Prob. 55EAPCh. 19 - Prob. 56EAPCh. 19 - Prob. 57EAPCh. 19 - .10 mol of nitrogen gas follow the two processes...Ch. 19 - Prob. 59EAPCh. 19 - Prob. 60EAPCh. 19 - Prob. 61EAPCh. 19 - Prob. 62EAPCh. 19 - Prob. 63EAPCh. 19 - Prob. 64EAPCh. 19 - Prob. 65EAPCh. 19 - Prob. 66EAPCh. 19 - Prob. 67EAPCh. 19 - Prob. 68EAPCh. 19 - Prob. 69EAPCh. 19 - A cylindrical copper rod and an iron rod with...Ch. 19 - Prob. 71EAPCh. 19 - Prob. 72EAPCh. 19 - Prob. 73EAPCh. 19 - Prob. 74EAPCh. 19 - Prob. 75EAPCh. 19 - Prob. 76EAPCh. 19 - Prob. 77EAPCh. 19 - Prob. 78EAPCh. 19 - Prob. 79EAPCh. 19 - Prob. 80EAPCh. 19 - Prob. 81EAPCh. 19 - Prob. 82EAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- One way to cool a gas is to let it expand. When a certain gas under a pressure of 5.00 106 Ha at 25.0C is allowed to expand to 3.00 times its original volume, its final pressure is 1.07 106 Pa. (a) What is the initial temperature of the gas in Kelvin? (b) What is the final temperature of the system? (See Section 10.4.)arrow_forward(a) How much heat must be added to raise the temperature of 1.5 mol of air 25.0 to 33.0 at constant volume? Assume air is completely diatomic. (b) Repeat the problem for the same number of moles of xenon, Xe.arrow_forward(a) The inside of a hollow cylinder is maintained at a temperature Ta, and the outside is at a lower temperature, Tb (Fig. P19.45). The wall of the cylinder has a thermal conductivity k. Ignoring end effects, show that the rate of energy conduction from the inner surface to the outer surface in the radial direction is dQdt=2Lk[TaTbln(b/a)] Suggestions: The temperature gradient is dT/dr. A radial energy current passes through a concentric cylinder of area 2rL. (b) The passenger section of a jet airliner is in the shape of a cylindrical tube with a length of 35.0 m and an inner radius of 2.50 m. Its walls are lined with an insulating material 6.00 cm in thickness and having a thermal conductivity of 4.00 105 cal/s cm C. A heater must maintain the interior temperature at 25.0C while the outside temperature is 35.0C. What power must be supplied to the heater? Figure P19.45arrow_forward
- Suppose 26.0 g of neon gas are stored in a tank at a temperature of 152C. (a) What is the temperature of the gas on the Kelvin scale? (See Section 10.2.) (b) How many moles of gas are in the tank? (See Section 10.4.) (c) What is the internal energy of the gas? (See Section 10.5.)arrow_forwardA glass windowpane in a home is 0.62 cm thick and has dimensions of 1.1 m × 1.7 m. On a certain day, the indoor temperature is 26°C and the outdoor temperature is 0°C. (a) What is the rate at which energy is transferred by heat through the glass? Answer in W (b) How much energy is lost through the window in one day, assuming the temperatures inside and outside remain constant? Answer in Jarrow_forwardLake Erie contains roughly 4.00 x 1011 m3 of water. (a) How much energy is required to raise the temperature of that volume of water from 11.0°C to 12.0°C? (b) How many years would it take to supply this amount of energy by using the 1.00 x 104-MW exhaust energy of an electric power plant?arrow_forward
- A typical person takes 12 breaths a minute, with each breath drawing in 0.500 L of outside air. If the air warms up from -10 °C to 30 °C. A. What is the volume of air exhaled with each breath? B. How many moles of air are exhaled? C. What is the heat transfer needed to warm each breath? Molar mass air = 29g/mol. Specific heat (air) = 721 J/(kg•°C) D. What is the rate of heat transfer?arrow_forwardA 1.0-m-long steel beam, initially at a temperature of 250 C, increases in temperature to 1000 C by inserting it into an insulating jacket for several minutes while the inside of the jacket is subsequently flooded with steam. By how much does the length of the steel beam expand? (The thermal coefficient of linear expansion for steel is 12 x 10-6 (C0)-1) a. 0.90 mm b. 1.0 mm c. 0.70 mm d. 0.80 mm e. 0.60 mmarrow_forwardA glass coffee pot has a circular bottom with a 9.00-cm diameter in contact with a heating element that keeps the coffee warm with a continuous heat transfer rate of 50.0 W (a) What is the temperature of the bottom of the pot, if it is 3.00 mm thick and the inside temperature is 60.0C? (b) If the temperature of the coffee remains constant and all of the heat transfer is removed by evaporation, how many grams per minute evaporate? Take the heat of vaporization to be 2340 kJ/kg.arrow_forward
- A. Before going in for an annual physical, a 70.0-kg person whose body temperature is 37.0∘C consumes an entire 0.355-liter can of a soft drink (which is mostly water) at 12.0∘C. B. What will be the person's body temperature Tfinal after equilibrium is attained? Ignore any heating by the person's metabolism. The specific heat capacity of a human body is 3480 J/kg⋅K. C. Is the change in the person's body temperature great enough to be measured by a medical thermometer? (A high-quality medical thermometer can measure temperature changes as small as 0.1∘C or less.) Yes or No?arrow_forwardA glass windowpane in a home is 0.62 cm thick and has dimensions of 1.0 m × 2.0 m.On a certain day, the indoor temperature is 25°C and the outdoor temperature is 0°C.(a) What is the rate at which energy is transferred by heat through the glass? (b) How much energy is lost through the window in one day, assuming the temperatures inside and outside remain constant? [6452 W]arrow_forwardA container holds 0.70 g of argon at a pressure of 7.5 atm. A) How much heat is required to increase the temperature by 100 oC at constant volume? (express the answer in joules). B) How much will the temperature increase if this amount of heat energy is transferred to the gas at constant pressure? (Express your answer in degrees Celsius.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY