
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Organic Chemistry (8th Edition)
8th Edition
ISBN: 9780134261430
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 27P
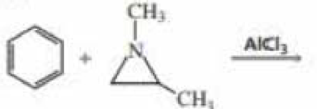
Benzene undergoes electrophilic aromatic substitution reactions with aziridines in the presence of a Lewis acid such as AlCl3.
- a. What are the major and minor products of the following reaction?
- b. Would you expect
epoxides to undergo similar reactions?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Br₂, PBrg
2. H₂O
H₂C
OH
H3C
OH
Br
The a-bromination of carbonyl compounds by Br₂ in acetic acid is limited to aldehydes and ketones because acids, esters,
and amides don't enolize to a sufficient extent. Carboxylic acids, however, can be a-brominated by first converting the
carboxylic acid to an acid bromide by treatment with PBr3. Following enolization of the acid bromide, Br₂ reacts in an α-
substitution reaction. Hydrolysis of the acid bromide completes the reaction.
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Arrow-pushing Instructions
H3C
:0:
:0::Br:
Br
Br
H3C
CO-P
H
Br
Br
Benzene undergoes electrophilic aromatic substitution reactions with aziridines in the presence of a Lewis acid such as AlCl3.a. What are the major and minor products of the following reaction?b. Would you expect epoxides to undergo similar reactions?
Rank the compounds according to their increasing reactivity toward electrophilic substitution.
1. Benzaldehyde
2. o-dimethylbenzene
3. nitrobenzene
4. phenol
Chapter 19 Solutions
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Organic Chemistry (8th Edition)
Ch. 19.1 - Name the following:Ch. 19.2 - Prob. 3PCh. 19.2 - Prob. 4PCh. 19.3 - Draw the product of each of the following...Ch. 19.5 - Prob. 6PCh. 19.5 - Explain why cyclopentadiene (pKa = 15) is more...Ch. 19.5 - When pyrrole is added to a dilute solution of...Ch. 19.6 - Prob. 10PCh. 19.6 - How to the mechanisms of the following reactions...Ch. 19.6 - Prob. 12P
Ch. 19.6 - Rank the following compounds from easiest to...Ch. 19.7 - Prob. 14PCh. 19.7 - Prob. 15PCh. 19.7 - Prob. 16PCh. 19.7 - Prob. 17PCh. 19.7 - Prob. 18PCh. 19.7 - Prob. 19PCh. 19.7 - Prob. 20PCh. 19 - Name the following:Ch. 19 - Prob. 22PCh. 19 - Rank the following compounds from strongest acid...Ch. 19 - Which of the following compounds is easier to...Ch. 19 - Rank the following compounds from most reactive to...Ch. 19 - One of the following compounds undergoes...Ch. 19 - Benzene undergoes electrophilic aromatic...Ch. 19 - Pyrrole reacts with excess...Ch. 19 - The dipole moments of furan and tetrahydrofuran...Ch. 19 - Name the following:Ch. 19 - Prob. 31PCh. 19 - Prob. 32PCh. 19 - a. Draw resonance contributors to show why...Ch. 19 - The chemical shifts of the C-2 hydrogen in the...Ch. 19 - Explain why protonating aniline has a dramatic...Ch. 19 - Prob. 36PCh. 19 - Propose a mechanism for the following reaction:Ch. 19 - Prob. 38PCh. 19 - Propose a mechanism for the following reactions:Ch. 19 - Prob. 40PCh. 19 - Prob. 41PCh. 19 - Prob. 42PCh. 19 - Organic chemists work with tetraphenylporphyrins...Ch. 19 - Show how the following compounds can be prepared...
Additional Science Textbook Solutions
Find more solutions based on key concepts
What is the pH range for acidic solutions? For basic solutions?
EBK INTRODUCTION TO CHEMISTRY
The method to determine the volume of a powered solid, liquid and a rock needs to be determined. Concept introd...
Living By Chemistry: First Edition Textbook
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Consider a sample of ideal gas initially in a volume V at temperature T and pressure P. Does the entropy of thi...
General Chemistry: Principles and Modern Applications (11th Edition)
Q2. Which statement best defines chemistry?
a. The science that studies solvents, drugs, and insecticides
b. Th...
Introductory Chemistry (5th Edition) (Standalone Book)
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Benzene undergoes electrophilic aromatic substitution reactions with aziridines in the presence of a Lewis acid such as AlCl3. a. What are the major and minor products of the following reaction? b. Would you expect epoxides to undergo similar reactions?arrow_forwardII. Alcohols in synthesis A. What are the strategies for the synthesis of alcohols by nucleophilic substitution reactions using halide leaving groups with hydroxide as a nucleophile? How do you predict possible by-products that could arise from this strategy? How do you use the less acetate nucleophile followed by treatment with NaOH to avoid by-products?arrow_forwardSynthesize the following compounds starting with benzene and any other inorganic reagents. 4 a. H2N Br b. O2N с.arrow_forward
- 7. How would you complete the following syntheses? Provide the reagents and products of each step. OH OH ⇒e" a. b. d. H Et OMearrow_forwardSynthesize each compound from benzene and any other organic or inorganic reagents. NH2 NO2 Br. a. е. NH2 Br CH3 Br NH2 b. d. HOOC NO2 (РАВA) sunscreen componentarrow_forwardEnols are quite reactive toward electrophiles than alkenes because: а. The OH group has a powerful electron-donating O resonance effect b. A resonance structure can be drawn that places a negative charge on one of the carbon atoms, making this carbon electrophilic С. The enol can react with nucleophilic carbon to form a new bond to carbon d. None of the options are correctarrow_forward
- Synthesize each compound from toluene and any other organic or inorganic reagents. a. C6HSCH2BR b. C6HSCH2OC(CH3)3 -CHO с. O,N НООС -NO2 d. H,N.arrow_forwardWhich one is an electrophile in the nitration of benzene? a) ΗNO b) NO2 c) NO3 d) ÑO i. a ii. d iii. iv.arrow_forwardRank the compounds in each group in order of increasing reactivity in nucleophilic acyl substitution.a. C6H5CO2CH3, C6H5COCl, C6H5CONH2 b. CH3CH2CO2H, (CH3CH2CO)2O, CH3CH2CONHCH3arrow_forward
- Wittig reactions with the following -chloroethers can be used for the synthesis of aldehydes and ketones. (a) Draw the structure of the triphenylphosphonium salt and Wittig reagent formed from each chloroether. (b) Draw the structural formula of the product formed by treating each Wittig reagent with cyclopentanone. Note that the functional group is an enol ether or, alternatively, a vinyl ether. (c) Draw the structural formula of the product formed on acid-catalyzed hydrolysis of each enol ether from part (b).arrow_forwardThe compound eutypine is an antibacterial agent isolated from the fungus Eutypa lata. This fungus results in a disease common to vineyards called eutyposis. Give a sequence of reactions that will take the following reactant and give eutypine when the other reactants used in the sequence are acetylene and acetone.arrow_forwardWrite out the expected splitting pattern for hydrogens Ha, Hp and Hc 3Jab = 10 Hz На Hb 3Jpc = 7 Hz CI Hc Hc Ha:Hb:Hc: doublet, quartet, doublet Ha:Hb:Hc: quartet, quartet, triplet Ha:Hb:Hc: doublet, doublet of triplets, doublet Ha:Hb:Hc: doublet, triplet of doublets, doubletarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY