
(a)
To sketch the cycle on PV diagram.
(a)
Explanation of Solution
Introduction:
The Brayton cycle consists of various thermodynamic process, an adiabatic compression, an isobaric expansion, an adiabatic expansion and an isobaric compression.
The figure below shows the PV curve of Brayton cycle,
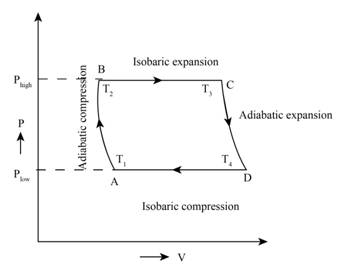
Figure (1)
From the range of temperature
Conclusion:
Therefore, the diagram of PV curve on Brayton cycle is shown in figure (1).
(b)
Show that the efficiency of the cycle as
(b)
Answer to Problem 83P
The efficiency of the cycle as
Explanation of Solution
Formula used:
The expression for the efficiency is,
Here, the heat absorbed from the heat reservoir during isobaric expansion is
The expression for change in enthalpy from first law of
The expression for
The expression for work done throught the cycle is,
Calculation:
The heat absorbed from the heat reservoir during isobaric expansion is calculated as,
By using the ideal gas law we get,
Similarly,
The delivered to cold reservoir during isobaric compression is calculated as
By using the ideal gas law we get
The efficiency is calculated as,
Conclusion:
Therefore, the efficiency of the cycle as
(c)
To show the efficiency is
(c)
Answer to Problem 83P
The efficiency is
Explanation of Solution
Formula used:
The expression for adiabatic process is given as,
Calculation:
The relation between pressure and temperature for adiabatic process AB is calculated as,
The relation between pressure and temperature for adiabatic process CD is calculated as,
Subtracting equation (1) with equation (2) we get,
By using efficiency from part (a) we get,
Conclusion:
Therefore, the efficiency is
Want to see more full solutions like this?
Chapter 19 Solutions
Physics for Scientists and Engineers, Vol. 3
- A monatomic ideal gas undergoes a quasi-static adiabatic expansion in which its volume is doubled. How is the pressure of the gas changed?arrow_forwardIs it possible for a system to have an entropy change if it neither absorbs nor emits heat during a reversible? transition? What happens it the process is irreversible?arrow_forwardThe energy output of a heat pump is greater than the energy used to operate the pump. Why doesn't this statement violate the first law of thermodynamics?arrow_forward
- Two moles of nitrogen gas, with =7/5 for ideal diatomic gases, occupies a volume of 102 m3 in an insulated cylinder at temperature 300 K. The gas is adiabatically and reversibly compressed to a volume of 5 L. The piston of the cylinder is locked in its place, and the insulation around the cylinder is removed. The heat-conducting cylinder is then placed in a 300-K bath. Heat from the compressed gas leaves the gas, and the temperature of the gas becomes 300 K again. The gas is then slowly expanded at the fixed temperature 300 K until the volume of the gas becomes 102 m3, thus making a complete cycle for the gas. For the entire cycle, calculate (a) the work done by the gas, (b) the heat into or out of the gas, (c) the change in the internal energy of the gas, and (d) the change in entropy of the gas.arrow_forwardA Carnot engine employs 1.5 mol of nitrogen gas as a working substance, which is considered as an ideal diatomic gas with =7.5 at the working temperatures of the engine. The Carnot cycle goes in the cycle ABCDA with AB being an isothermal expansion. The volume at points A and C of the cycle are 5.0103 m3 and 0.15 L, respectively. The engine operates between two thermal baths of temperature 500 K 300 K. (a) Find the values of volume at B and D. (b) How much heat is absorbed by the gas in the AB isothermal expansion? (c) How much work is done by the gas in the AB isothermal expansion? (d) How much heat is given up by the gas in the CD isothermal expansion? (e) How much work is done by the gas in the CD isothermal compression? (f) How much work is done by the gas in the BC adiabatic expansion? (g) How much work is done by the gas in the DA adiabatic compression? (h) Find the value of efficiency of the engine based on the net and heat input. Compare this value to the efficiency of a Carnot engine based on the temperatures of the baths.arrow_forwardThe figure shows a reversible cycle through which 1.00 mole of a monatomic ideal gas is taken. Process bc is an adiabatic expansion, with pb = 5.80 atm and Vb = 1.00 x 10-3 m3. For the cycle, find (a) the energy added to the gas as heat, (b) the energy leaving the gas as heat, (c) the net work done by the gas, and (d) the efficiency of the cycle.arrow_forward
- Compare the charge in the internal energy of an ideal gas for a quasi-static adiabatic expansion with that for a quasi-static isothermal expansion. What happens to the temperature of an ideal gas in an adiabatic expansion?arrow_forwardDuring the compression stroke of a certain gasoline engine, the pressure increases from 1.00 atm to 20.0 atm. If the process is adiabatic and the air–fuel mixture behaves as a diatomic ideal gas, (a) by what factor does the volume change and (b) by what factor does the temperature change? Assuming the compression starts with 0.016 0 mol of gas at 27.0°C, find the values of (c) Q, (d) ΔEint, and (e) W that characterize the process.arrow_forwardCalculate the heat Q produced during the isobaric cooling process of ideal gas sample with adiabatic coefficient γ of 1.4, if the work W done by the compression is 16 J.arrow_forward
- As shown in the figure, a chamber with a moveable piston and containing a monatomic ideal gas in an initial state A undergoes an isovolumetric, then an isothermal, and finally an isobaric process to complete the cycle. When the gas is in the initial state, the volume is 3.00 L, the pressure is 5.00 atm, and the temperature is 200 K. The gas is first warmed at constant volume to a pressure of 4 times the initial value (state B). The gas is then allowed to expand isothermally to some new volume (state C). Finally, it is compressed isobarically to its initial state. Due to the nature of this problem, do not use rounded intermediate values in your calculations. Determine values (in kJ) for Q, W, and ΔEint for the process C → A. Determine values (in kJ) for Q, W, and ΔEint for the complete cycle A → B → C → A.arrow_forwardAs shown in the figure, a chamber with a moveable piston and containing a monatomic ideal gas in an initial state A undergoes an isovolumetric, then an isothermal, and finally an isobaric process to complete the cycle. When the gas is in the initial state, the volume is 3.00 L, the pressure is 5.00 atm, and the temperature is 200 K. The gas is first warmed at constant volume to a pressure of 4 times the initial value (state B). The gas is then allowed to expand isothermally to some new volume (state C). Finally, it is compressed isobarically to its initial state. Due to the nature of this problem, do not use rounded intermediate values in your calculations. Find the volume of the gas at state C (in L). Determine values (in kJ) for Q, W, and ΔEint for the process A → B. Determine values (in kJ) for Q, W, and ΔEint for the process B → C.arrow_forwardRepeat the preceding calculations for an ideal diatomic gas expanding adiabatically from an initial volume of 0.500 m3 to a final volume of 1.25 m3, starting at a pressure of 1.01 105 Pa. (You must sketch the curve to find the work.) P2 = PaW ≈ Jarrow_forward

 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning


