
Concept explainers
Carvone is the major constituent of spearmint oil. What products would you expect from reaction of carvone with the following reagents?
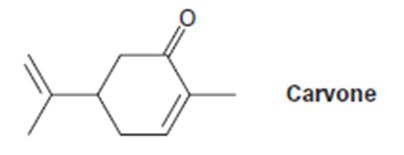
a) (CH3)2CU-Li+, then H3O+
b) LiA1H4, then H3O+
c) CH3NH2
d) C6H5MgBr, then H3O+
e) H2/Pd
f) CrO3, H3O+
g) (C6H5)3 P+C- HCH3
h) HOCH2CH2OH, HC1
a)
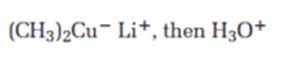
Interpretation:
The product expected when carvone reacts first with (CH3)2Cu- Li+ and then with H3O+ is to be stated.
Concept introduction:
α, β- Unsaturated ketones undergo conjugate (1,4-addition) addition reaction when treated with lithium diorganocopper reagents to yield saturated ketones as the product. Any primary, secondary, tertiary alkyl, alkenyl, and aryl halides can be used to prepare the lithium diorganocopper reagents.
To state:
The product expected when carvone reacts first with (CH3)2Cu- Li+ and then with H3O+.
Answer to Problem 64AP
The product expected when carvone reacts first with (CH3)2Cu- Li+ and then with H3O+ is
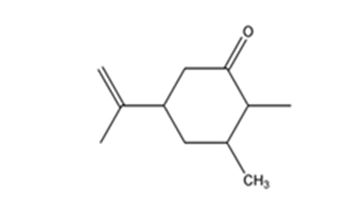
Explanation of Solution
Carvone is an α, β- unsaturated ketone. When treated with (CH3)2Cu- Li+ it undergoes a conjugate addition to yield the corresponding saturated ketone.
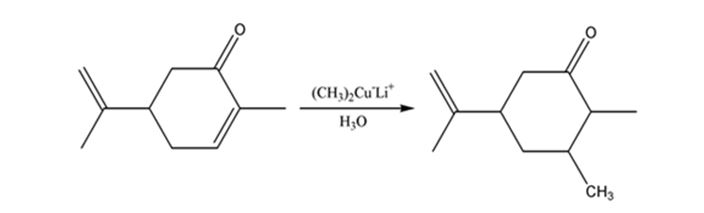
The product expected when carvone reacts first with (CH3)2Cu- Li+ and then with H3O+ is

b)
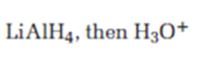
Interpretation:
The product expected when carvone reacts first with LiAlH4 and then with H3O+ is to be stated.
Concept introduction:
Unsaturated ketones can be reduced to secondary alcohols without affecting the double bond by treating with LiAlH4 and then with H3O+.
To state:
The product expected when carvone reacts first with LiAlH4 and then with H3O+.
Answer to Problem 64AP
The product expected when carvone reacts first with LiAlH4 and then with H3O+ is
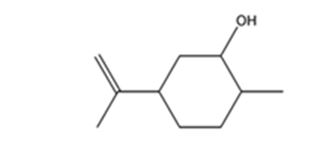
Explanation of Solution
Carvone is an α, β- unsaturated ketone. LiAlH4 reduces it to a secondary alcohol. The double bond remains unaffected during the reduction.
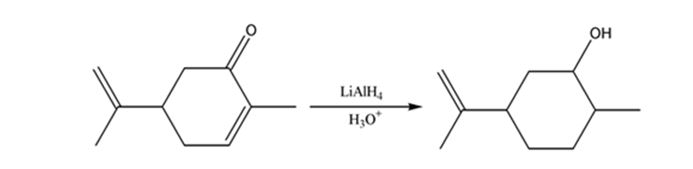
The product expected when carvone reacts first with LiAlH4 and then with H3O+ is

c)
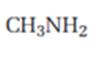
Interpretation:
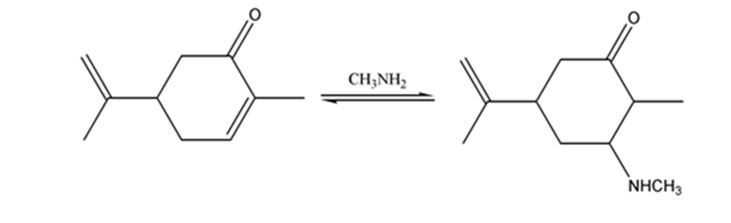
The product expected when carvone reacts with CH3NH2 is to be stated.
Concept introduction:
Both primary and secondary amines react with α, β- unsaturated ketones to yield β-aminoaldehydes and ketones by conjugate addition.
To state:
The product expected when carvone reacts with CH3NH2.
Answer to Problem 64AP
The product expected when carvone reacts with CH3NH2 is
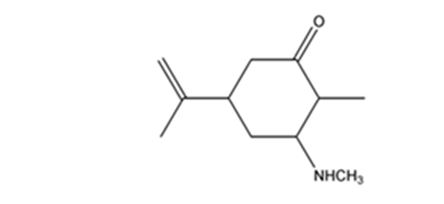
Explanation of Solution
Carvone is an α, β- unsaturated ketone. When treated with methyl amine it undergoes conjugate addition to yield the β-N-methylamino ketone as the product.
The product expected when carvone reacts with CH3NH2 is

d)

Interpretation:
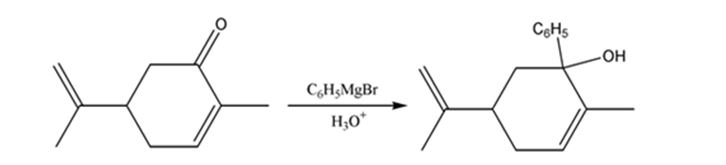
The product expected when carvone reacts first with C6H5MgBr and then with H3O+ is to be stated.
Concept introduction:
Ketones undergo a 1,2-addition reaction with Grignard reagent to give an intermediate which upon acidification yields tertiary alcohols.
To show:
The product expected when carvone reacts first with C6H5MgBr and then with H3O+.
Answer to Problem 64AP
The product expected when carvone reacts first with C6H5MgBr and then with H3O+ is
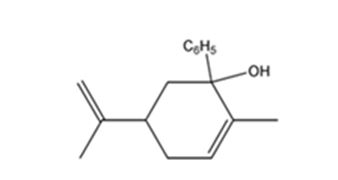
Explanation of Solution
Carvone is an α, β- unsaturated ketone. When treated with C6H5MgBr, the Grignard reagent adds to the carbonyl group to yield an intermediate which when treated with aqueous acids yield the tertiary alcohol required.
The product expected when carvone reacts first with C6H5MgBr and then with H3O+ is

e)
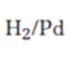
Interpretation:
The product expected when carvone reacts with H2/Pd is to be stated.
Concept introduction:
Hydrogen in the presence of Pd reduces the double and triple bonds in organic molecules. It does not reduce the carbonyl group in aldehydes and ketones.
To state:
The product expected when carvone reacts with H2/Pd.
Answer to Problem 64AP
The product expected when carvone reacts with H2/Pd is
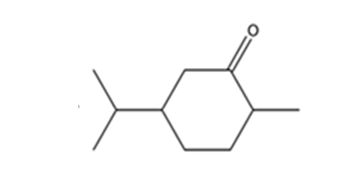
Explanation of Solution
Carvone is an α, β- unsaturated ketone. When treated with hydrogen in the presence of Pd the double bond in it is reduced and the C= O group in it remains unaffected.
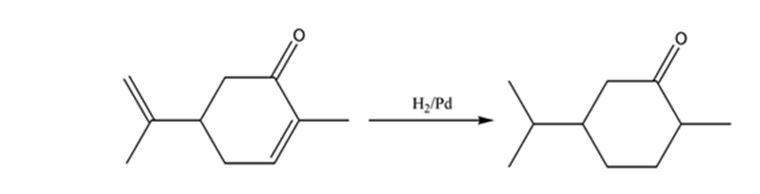
The product expected when carvone reacts with H2/Pd is

f)
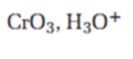
Interpretation:
The product expected when carvone reacts with CrO3, H3O+ is to be stated.
Concept introduction:
Ketones are not oxidized by CrO3, H3O+.
To state:
The product expected when carvone reacts with CrO3, H3O+.
Answer to Problem 64AP
Carvone does not reacts with CrO3, H3O+.
Explanation of Solution
Carvone is an α, β- unsaturated ketone. It is not oxidized by CrO3, H3O+.
Carvone does not reacts with CrO3, H3O+.
g)
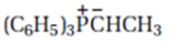
Interpretation:
The product expected when carvone reacts with (C6H5)3P+CH- CH3 is to be stated.
Concept introduction:
When ketones are treated with phosphoranes, an exchange the O in the ketone and alkyl group in phosphorane takes place to yield an alkene and phosphorus oxide as the products.
To state:
The product expected when carvone reacts with (C6H5)3P+CH- CH3.
Answer to Problem 64AP
The product expected when carvone reacts with (C6H5)3P+CH- CH3 is
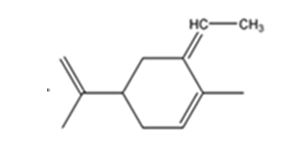
The compound exists as E and Z isomers.
Explanation of Solution
The given reaction is a Wittig reaction. When the ketone, carvone, is treated with the phosphorane, an exchange of oxygen in it and alkyl group in phosphorane takes place to yield an alkene as the product. The alkene exhibits geometrical isomerism and exists as E and Z isomers.
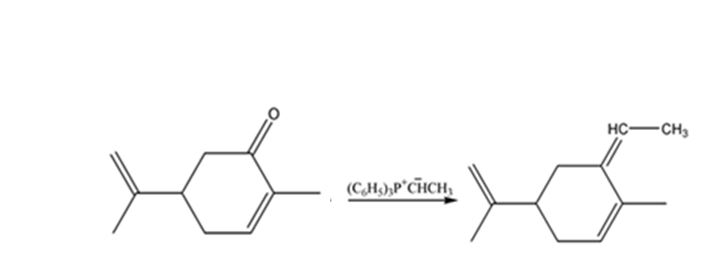
The product expected when carvone reacts with (C6H5)3P+CH- CH3 is

The compound exists as E and Z isomers.
h)
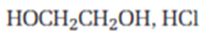
Interpretation:
The product expected when carvone reacts with HOCH2CH2OH is to be stated.
Concept introduction:
Aldehydes and ketones, when treated with dihydric alcohols in the presence of acid catalyst yield cyclic acetals as products.
To state:
The product expected when carvone reacts with HOCH2CH2OH.
Answer to Problem 64AP
The product expected when carvone reacts with HOCH2CH2OH is
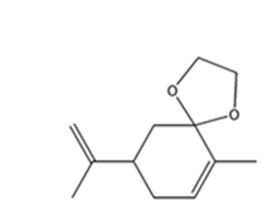
Explanation of Solution
Carvone is an α, β- unsaturated ketone. Hence when treated with glycol, a dihydric alcohol, in the presence of HCl it yields a cyclic acetal as product.
The product expected when carvone reacts with HOCH2CH2OH is

Want to see more full solutions like this?
Chapter 19 Solutions
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- A step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Propose a mechanism for formation of this bromolactone and account for the observed stereochemistry of each substituent on the cyclohexane ring. Alprostadil is used as a temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation.arrow_forwardAmines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forwardCembrene, C20H32, is a diterpenoid hydrocarbon isolated from pine resin. Cembrene has a UV absorption at 245 nm, but dihydrocembrene (C20H34), the product of hydrogenation with 1 equivalent of H2, has no UV absorption. On exhaustive hydrogenation, 4 equivalents of H2 react, and octahydrocembrene, C20H40, is produced. On ozonolysis of cembrene, followed by treatment of the ozonide with zinc, four carbonylcontaining products are obtained: Propose a structure for cembrene that is consistent with its formation from geranylgeranyl diphosphate.arrow_forward
- Reaction of (Z)-methyl-3-hexene with H2O/H2SO4 produces 3-methyl-3-hexanol (75% yield) 1. Write a balance chemical equation for the reaction. 2. Propose a reaction mechanistic explanation for the reaction.arrow_forward7. When butan-2-ol undergoes acid catalysed dehydration reaction, a mixture of products, B and C, are formed.Compound C shows cis-trans isomerism. Ozonolysis of compound B yields D and E. Draw the structure of Band C, and determine the major product. Draw cis-trans isomers of C. Write the reaction equation for theozonolysis of compound B that yields D and E. Show the preparation of butan-2-ol via a Grignard reagent byusing a suitable alkyl halide and compound D and E.arrow_forwardReaction of N,N-diethyl-p-diaminobenzene with sodium nitrite and hydrochloric acid at 0°C and subsequent reaction with nitrobenzene.arrow_forward
- What is the best set of reagents to achieve deoxygenation of 2-pentanone to pentane? A. NaClO2/NaH2PO4 B. LiAlH4, Et2O C. DIBAL-H, THF D. NH2NH2/t-BuOK, DMSOarrow_forwardDraw the organic products formed when cyclopentene is treated with each reagent. With some reagents, no reactionoccurs.a. H2 + Pd-Cb. H2 + Lindlar catalystc. Na, NH3d. CH3CO3He. [1] CH3CO3H; [2] H2O, HO–f. [1] OsO4 + NMO; [2] NaHSO3, H2Og. KMnO4, H2O, HO–h. [1] LiAlH4; [2] H2Oi. [1] O3; [2] CH3SCH3j. (CH3)3COOH, Ti[OCH(CH3)2]4, (–)-DETk. mCPBAl. Product in (k); then [1] LiAlH4; [2] H2Oarrow_forwardA. In the synthesis of 1-bromobutane, what is the inorganic by-product left in the reaction flask following the distillation? Why was the bromoalkane the bottom layer in the separatory funnel? B. Predict the product when 1-methylcyclohexanol reacts with H2SO4 and KBr. Show the mechanism.arrow_forward
- Choose the right reagent or series of reagents from the ones listed below to prepare 2-pentanone from acetylene. NaNH2NaNH2 followed by CH3CH2CH2BrCH3CH2CH2Br, then disiamylborane followed by H2O2H2O2, HO−HO− H2H2, Lindlar followed by H2OH2O, H+H+ NaNH2NaNH2 and CH3CH2CHOCH3CH2CHO NaNH2NaNH2 followed by H2OH2O, H+H+ NaNH2NaNH2 followed by CH3CH2CH2BrCH3CH2CH2Br, then H2OH2O, H2SO4H2SO4, HgSO4 Choose one.arrow_forwardBenzene is one of the compounds used as octane enhancers in unleaded gasoline. It is manufactured by thecatalytic conversion of acetylene to benzene: 3C2 H2(g) ⇌ C6 H6(g). Which value of Kc would make this reactionmost useful commercially? Kc ≈ 0.01, Kc ≈ 1, or Kc ≈ 10. Explain your answerarrow_forwardA certain compound of molecular formula C19H38 was isolated from fish oil and from plankton. On hydrogenation it gave 2,6,10,14-tetramethylpentadecane. Ozonolysis gave (CH3)2C O and a 16-carbon aldehyde. What is the structure of the natural product? What is the structure of the aldehyde?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

