
Chemistry: The Central Science Plus Mastering Chemistry, 13th Edition
13th Edition
ISBN: 9780321864406
Author: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 105AE
Suppose you had a balloon made of some highly flexible semipermeable membrane. The balloon is filled completely with a 0.2 M solution of some solute and is submerged in a 0.1 M solution of the same solute:

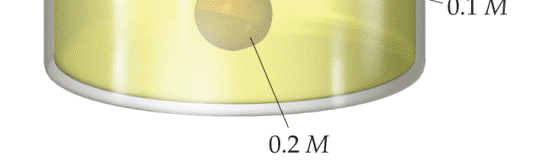
Initially, the volume of solution in the balloon is 0.25 L. Assuming the volume outside the semipermeable membrane is large, as the illustration shows, what would you expect for the solution volume inside the balloon once the system has come to equilibrium through osmosis? [Section 13.5]
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Chemistry: The Central Science Plus Mastering Chemistry, 13th Edition
Ch. 2.3 - Prob. 2.1.1PECh. 2.3 - Prob. 2.1.2PECh. 2.3 - Prob. 2.2.1PECh. 2.3 - Prob. 2.2.2PECh. 2.3 - Prob. 2.3.1PECh. 2.3 - Prob. 2.3.2PECh. 2.4 - Practice Exercise 1 The atomic weight of copper,...Ch. 2.4 - Prob. 2.4.2PECh. 2.5 - Prob. 2.5.1PECh. 2.5 - Prob. 2.5.2PE
Ch. 2.6 - 11.93 The vapor pressure of ethanol (C2H5OH) at 19...Ch. 2.6 - Prob. 2.6.2PECh. 2.7 - Prob. 2.7.1PECh. 2.7 - Prob. 2.7.2PECh. 2.7 - Prob. 2.8.1PECh. 2.7 - Consider the two-dimensional square lattice of...Ch. 2.7 - Prob. 2.9.1PECh. 2.7 - Given the ionic radii and molar masses of Sc3+...Ch. 2.7 - Prob. 2.10.1PECh. 2.7 - Prob. 2.10.2PECh. 2.8 - Prob. 2.11.1PECh. 2.8 - Prob. 2.11.2PECh. 2.8 - Prob. 2.12.1PECh. 2.8 - Prob. 2.12.2PECh. 2.8 - Prob. 2.13.1PECh. 2.8 - The table below shows the normal boiling points of...Ch. 2.8 - Prob. 2.14.1PECh. 2.8 - Prob. 2.14.2PECh. 2.9 - Prob. 2.15.1PECh. 2.9 - Prob. 2.15.2PECh. 2 - Prob. 1ECh. 2 - At 280C, raw milk sours in 4.0 h but takes 48 h to...Ch. 2 - At 900 o C, Kc = 0.0108 for the reaction CaCO3(g) ...Ch. 2 - Calculate the molar concentration of OH- in a...Ch. 2 - Pyridinium bromide (C5H5NHBr) is a strong...Ch. 2 - Prob. 6ECh. 2 - Prob. 7ECh. 2 - Prob. 8ECh. 2 - Prob. 9ECh. 2 - Indicate whether each statement is true or false:...Ch. 2 - Prob. 11ECh. 2 - Prob. 12ECh. 2 - Prob. 13ECh. 2 - At 20 oC, the vapor pressure of benzene (C6 H6) is...Ch. 2 - Summarize the evidence used by J. J. Thomson to...Ch. 2 - Prob. 16ECh. 2 - Prob. 17ECh. 2 - Prob. 18ECh. 2 - Suppose the rate law for the reaction in this...Ch. 2 - Practice Exercise 1 Using the data in Sample...Ch. 2 - Which of the following linear plots do you expect...Ch. 2 - A flask is charged with 0.100 mol of A and allowed...Ch. 2 - Prob. 23ECh. 2 - Prob. 24ECh. 2 - Prob. 25ECh. 2 - Prob. 26ECh. 2 - The addition of No accelerates the decomposition...Ch. 2 - Prob. 28ECh. 2 - Prob. 29ECh. 2 - The rates of many atmospheric reactions are...Ch. 2 - Prob. 31ECh. 2 - Prob. 32ECh. 2 - Prob. 33ECh. 2 - 15.23 The equilibrium constant for the...Ch. 2 - A mixture of 0.10 mol of NO, 0.050 mol of H2, and...Ch. 2 - Prob. 36ECh. 2 - Prob. 37ECh. 2 - Prob. 38ECh. 2 - Prob. 39ECh. 2 - Prob. 40ECh. 2 - Practice Exercise 1 Order the following three...Ch. 2 - Practice Exercise 1 What is the pH of a 0.28 M...Ch. 2 - Prob. 43ECh. 2 - Which of the following diagrams best represent an...Ch. 2 - Prob. 45ECh. 2 - Prob. 46ECh. 2 - Prob. 47ECh. 2 - Prob. 48ECh. 2 - Prob. 49ECh. 2 - 16.72 Calculate the molar concentration of OH- in...Ch. 2 - Prob. 51ECh. 2 - Prob. 52ECh. 2 - Prob. 53ECh. 2 - Prob. 54ECh. 2 - a. Given that Ka for acetic acid is 1.8 10-5 and...Ch. 2 - 16.78
a. Given that Kb for ammonia is 1.8 X 10 -5...Ch. 2 - Prob. 57ECh. 2 - Prob. 58ECh. 2 - Prob. 59ECh. 2 - Prob. 60ECh. 2 - Prob. 61ECh. 2 - Prob. 62ECh. 2 - 16.86 An unknown salt is either KBr, NH4 C1, KCN,...Ch. 2 - Prob. 64ECh. 2 - Prob. 65ECh. 2 - 16.89 Based on their compositions and structures...Ch. 2 - Prob. 67ECh. 2 - 16.91 Indicate whether each of the following...Ch. 2 - Prob. 69ECh. 2 - Prob. 70ECh. 2 - Prob. 71ECh. 2 - Prob. 72ECh. 2 - Prob. 73ECh. 2 - Prob. 74ECh. 2 - Prob. 75ECh. 2 - Prob. 76ECh. 2 - Prob. 77ECh. 2 - Prob. 78ECh. 2 - Prob. 79ECh. 2 - Benzoic acid (C6H5COOH) and aniline (C6H5NH2) are...Ch. 2 - Prob. 81ECh. 2 - Prob. 82ECh. 2 - Prob. 83ECh. 2 - Butyric acid is responsible for the foul smell of...Ch. 2 - Prob. 85ECh. 2 - Prob. 86ECh. 2 - Prob. 87AECh. 2 - 1S.113 Many moderately large organic molecules...Ch. 2 - Prob. 89AECh. 2 - Prob. 90AECh. 2 - Prob. 91AECh. 2 - Prob. 92AECh. 2 - Prob. 93AECh. 2 - 16.120 At 50 oC, the ion-product constant for H2...Ch. 2 - Prob. 95AECh. 2 - Prob. 96AECh. 2 - Prob. 97AECh. 2 - Prob. 98AECh. 2 - Prob. 99AECh. 2 - Which two statements about gas mixtures are true?...Ch. 2 - Prob. 101AECh. 2 - 13.6 If you compare the solubilities of the noble...Ch. 2 - Prob. 103AECh. 2 - Prob. 104AECh. 2 - Suppose you had a balloon made of some highly...Ch. 2 - Prob. 106AECh. 2 - Indicate whether each statement is true or false:...Ch. 2 - Indicate the type of solute-solvent interaction...Ch. 2 - An ionic compound has a very negative H soln in...Ch. 2 - Prob. 110AECh. 2 - Prob. 111AECh. 2 - The solubility of Cr (NO3)3 . 9 H2O in water is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A patient has a “cholesterol count” of 214. Like manyblood-chemistry measurements,this result is measured inunits of milligrams per deciliter (mgdL1). Determine the molar concentration of cholesterol inthis patient’s blood, taking the molar mass of cholesterolto be 386.64gmol1. Estimate the molality of cholesterol in the patient’sblood. If 214 is a typical cholesterol reading among men inthe United States, determine the volume of such bloodrequired to furnish 8.10 g of cholesterol.arrow_forwardSolutions Introduced directly into the bloodstream have to be isotonic with blood; that is, they must have the same osmotic pressure as blood. An aqueous NaCl solution has to be 0.90% by mass to be isotonic with blood. What is the molarity of the sodium ions in solution? Take the density of the solution to be 1.00 g/mL.arrow_forwardExplain why the distinction between solute and solvent is not clear for some solutions.arrow_forward
- You have read that adding a solute to a solvent can both increase the boiling point and decrease the freezing point. A friend of yours explains it to you like this: The solute and solvent can be like salt in water. The salt gets in the way of freezing in that it blocks the water molecules from joining together. The salt acts like a strong bond holding the water molecules together so that it is harder to boil. What do you say to your friend?arrow_forwardA pharmacist prepares an isotonic saline solution for intravenous infusion. Instead of preparing a 0.15 M solution, a 1.5 M solution is prepared. What would happen to the red blood cells if this erroneously prepared solution is infused?arrow_forwardInsulin is a hormone responsible for the regulation of glucose levels in the blood. An aqueous solution of insulin has an osmotic pressure of 2.5 mm Hg at 25C. It is prepared by dissolving 0.100 g of insulin in enough water to make 125 mL of solution. What is the molar mass of insulin?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY