
Concept explainers
(a)
To identify: The potential hydrogen bond donors and accepters present in Fig.1.
Concept introduction: Hydrogen bonds are formed between polar molecules. It is an intermolecular attraction that forms between partially positive hydrogen atoms of a polar molecule with a partially negative atom of another polar molecule. In general chemical formulation, a hydrogen bond is explained as D–H···A, where D–H is a hydrogen bond “donor group” and A is considered as a hydrogen bond accepter group or an atom.
(a)
Answer to Problem 1E
Correct answer: The potential hydrogen bond donor groups are HN1, H2N at the C2 position, and HN9. The potential hydrogen bond accepter groups are O at the C6 position, N3, and N7.
Explanation of Solution
Pictorial presentation: Fig. 1 shows structure of guanine, where the potential hydrogen bond donors and accepters are identified.
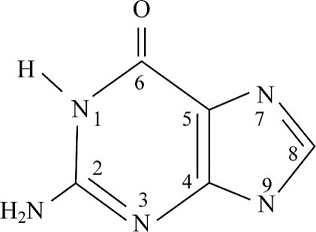
Fig.1: Guanine
The given formula of Fig.1 is identified as Guanine. Here, at the HN1 position, the hydrogen bond is donated and nitrogen acts as a hydrogen bond donor when it is paired with a hydrogen atom. When nitrogen is not paired with a hydrogen atom, it acts as a hydrogen accepter. Therefore, at HN1, H2N at the C2 position, and HN9 portion, the hydrogen bond acts like a donor; O at the C6 position, N3, and N7 portion, the hydrogen bond acts like an acceptor.
(b)
To identify: The potential hydrogen bond donors and acceptors present in Fig.2.
Concept introduction: Hydrogen bonds are formed between polar molecules. It is an intermolecular attraction that forms between partially positive hydrogen atoms of a polar molecule with a partially negative atom of another polar molecule. In general chemical formulation, a hydrogen bond is explained as D–H···A, where D–H is a hydrogen bond “donor group” and A is considered as a hydrogen bond accepter group or atom.
(b)
Answer to Problem 1E
Correct answer: The potential hydrogen bond donor groups are HN1 and H2N at the C4 position. The potential hydrogen bond accepter groups are O at the C2 position, N3.
Explanation of Solution
Pictorial presentation: Fig.2 shows structure of cytosine, where the potential hydrogen bond donors and accepters are identified.
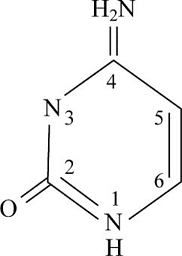
Fig.1: Cytosine
The given formula of Fig.2 is identified as cytosine. Here, at HN1 position, the hydrogen bond is donated as nitrogen acts as the hydrogen bond donor when it is paired with a hydrogen atom. When nitrogen is not paired with a hydrogen atom, it acts as a hydrogen acceptor. Therefore, at HN1 and H2N at the C4 position, the hydrogen bond acts like a donor and O at the C2 position and N3 portion, the hydrogen bond acts like an acceptor.
(c)
To identify: The potential hydrogen bond donors and accepters present in Fig.3.
Concept introduction: Hydrogen bonds are formed between the polar molecules. It is an intermolecular attraction that forms between partially positive hydrogen atoms of a polar molecule with a partially negative atom of another polar molecule. In general chemical formulation, a hydrogen bond is explained as D–H···A, where D–H is a hydrogen bond “donor group” and A is considered as a hydrogen bond accepter group or atom.
(c)
Answer to Problem 1E
Correct answer: The potential hydrogen bond donor groups are H3N+ group and OH group. The potential hydrogen bond accepter groups are COO and OH.
Explanation of Solution
Pictorial presentation: Fig.3 shows structure of serine, where the potential hydrogen bond donors and acceptors are identified.
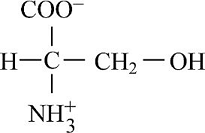
Fig.3: Serine
The given formula of Fig.3 is identified as serine. Here, at H3N+ position, the hydrogen bond is donated as nitrogen acts as a hydrogen bond donor when it is paired with a hydrogen atom. When nitrogen is not paired with a hydrogen atom, it acts as a hydrogen acceptor. Therefore, at H3N+ group and OH group, the hydrogen bond acts like a donor and COO and OH position, the hydrogen bond acts like an acceptor.
Want to see more full solutions like this?
Chapter 2 Solutions
Fundamentals Of Biochemistry