
The curve shown in Figure 2-1 is typical of a reaction carried out isothermally, and the curve shown in Figure P2-10B (sec Example 11-3) is typical of a gas-solid catalytic exothermic reaction carried out adiabatically).
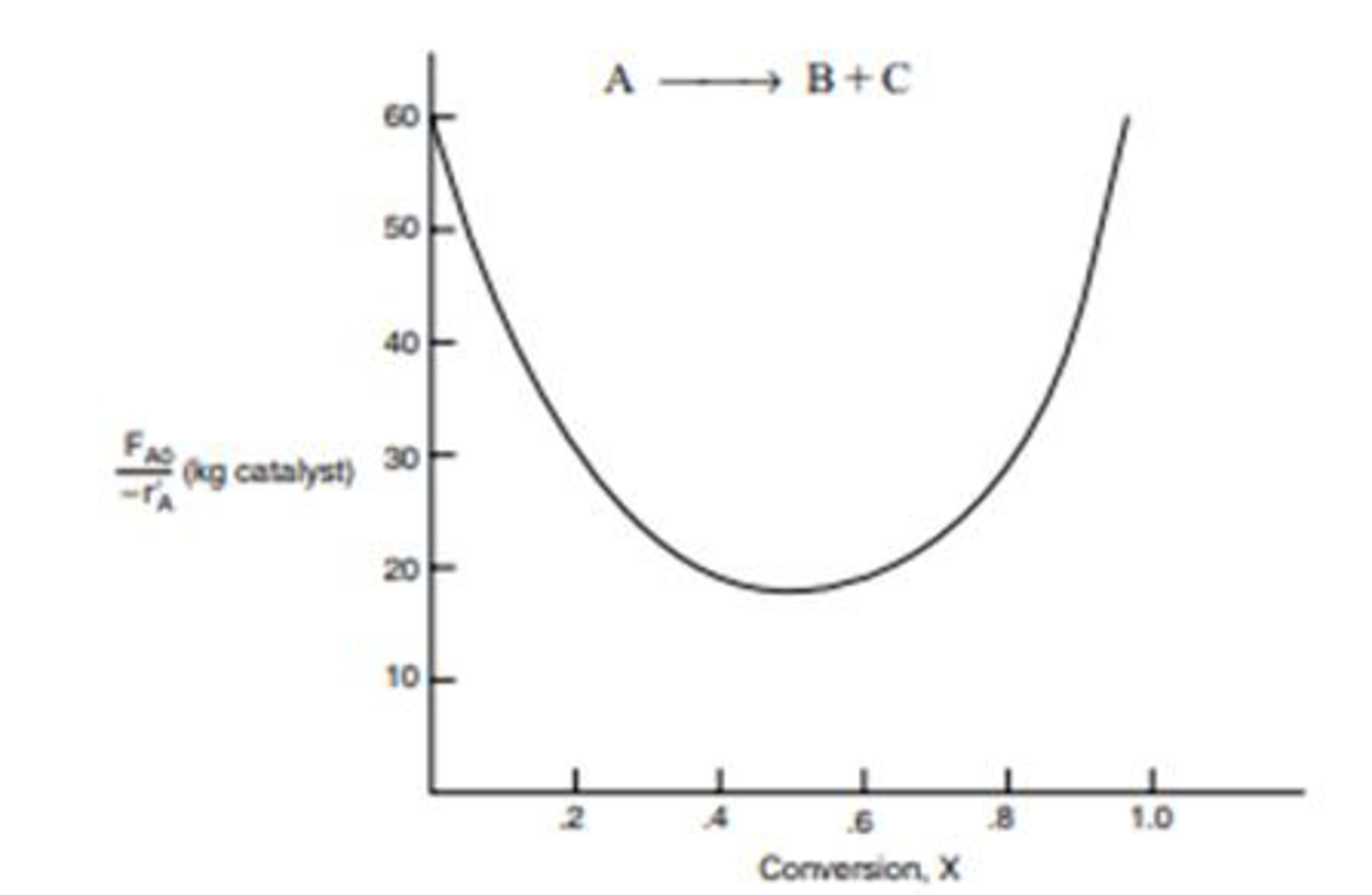
Figure P2-10B Levenspiel plot for an adiabatic exothermic heterogeneous reaction.
- (a) Assuming that you have a fluidized CSTR and a PBR containing equal weights of catalyst, how should they be arranged for this adiabatic reaction? Use the smallest amount of catalyst weight to achieve 80% conversion of A.
- (b) What is the catalyst weight necessary to achieve 80% conversion in a fluidized CSTR?
- (c) What fluidized CSTR weight is necessary to achieve 40% conversion?
- (d) What PBR weight is necessary to achieve 80% conversion?
- (e) What PBR weight is necessary to achieve 40% conversion?
- (f) Plot the rate of reaction and conversion as a function of PBR catalyst weight, W.
Additional information: FA0 = 2 mol/s.
Learn your wayIncludes step-by-step video

Chapter 2 Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Additional Engineering Textbook Solutions
Starting Out with C++ from Control Structures to Objects (9th Edition)
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Engineering Mechanics: Statics
Engineering Mechanics: Dynamics (14th Edition)
Web Development and Design Foundations with HTML5 (8th Edition)
Modern Database Management (12th Edition)
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





